Text Solution
Verified by Experts
Topper's Solved these Questions
HEAT
PEARSON IIT JEE FOUNDATION|Exercise LEVEL 3|10 VideosHEAT
PEARSON IIT JEE FOUNDATION|Exercise TEST YOUR CONCEPTS (Very Short Answer Type Questions)|24 VideosHEAT
PEARSON IIT JEE FOUNDATION|Exercise LEVEL 1 MCQ s|30 VideosFRICTION
PEARSON IIT JEE FOUNDATION|Exercise Competition Corner |54 VideosHYDROSTATICS
PEARSON IIT JEE FOUNDATION|Exercise CONCEPT APPLICATION (LEVEL 3)|10 Videos
Similar Questions
Explore conceptually related problems
PEARSON IIT JEE FOUNDATION-HEAT-LEVEL 2
- The density of two spheres of equal radius are in the ratio 1:3 and th...
Text Solution
|
- The total distance between the lower fixed point and the upper fiixed ...
Text Solution
|
- When the mercury thread rises to 3/4th of the distance between the two...
Text Solution
|
- On a certain scale of temperature, the freezing and boiling points of ...
Text Solution
|
- Two cylinderical bodies 'A' and 'B' have their radii in the ratio of 1...
Text Solution
|
- The rate at which ice melts is more at the top when compared to the bo...
Text Solution
|
- The density of two identical spheres are in the ratio 2:3 and their sp...
Text Solution
|
- In a new scale of temperature the lower fixed point is marked as 0 cor...
Text Solution
|
- How is water useful in the protection of fruits and vegetables from da...
Text Solution
|
- Two substances P and Q are heated by using similar heating devices. Th...
Text Solution
|
- Why is salt sprinkled under ice cube trays in a refrigerator?
Text Solution
|
- The temperature of a body is measured in Kelvin scale, Fahrenheit scal...
Text Solution
|
- It is possible to melt a piece of aluminium placed in a spoon made of ...
Text Solution
|
- A new scale fo temperature is introduced. One degree temperature diffe...
Text Solution
|
- Convert 55^(@)C into Fahrenheit and Kelvin scale.
Text Solution
|
- Three metallic spheres A,B and C have their masses in the ratio 1:2:3,...
Text Solution
|
- If 3360 J of heat is required to melt 10 g of ice, how many kilocalori...
Text Solution
|
- Find the amount of heat enegy required to convert 100 g of ice at -10^...
Text Solution
|
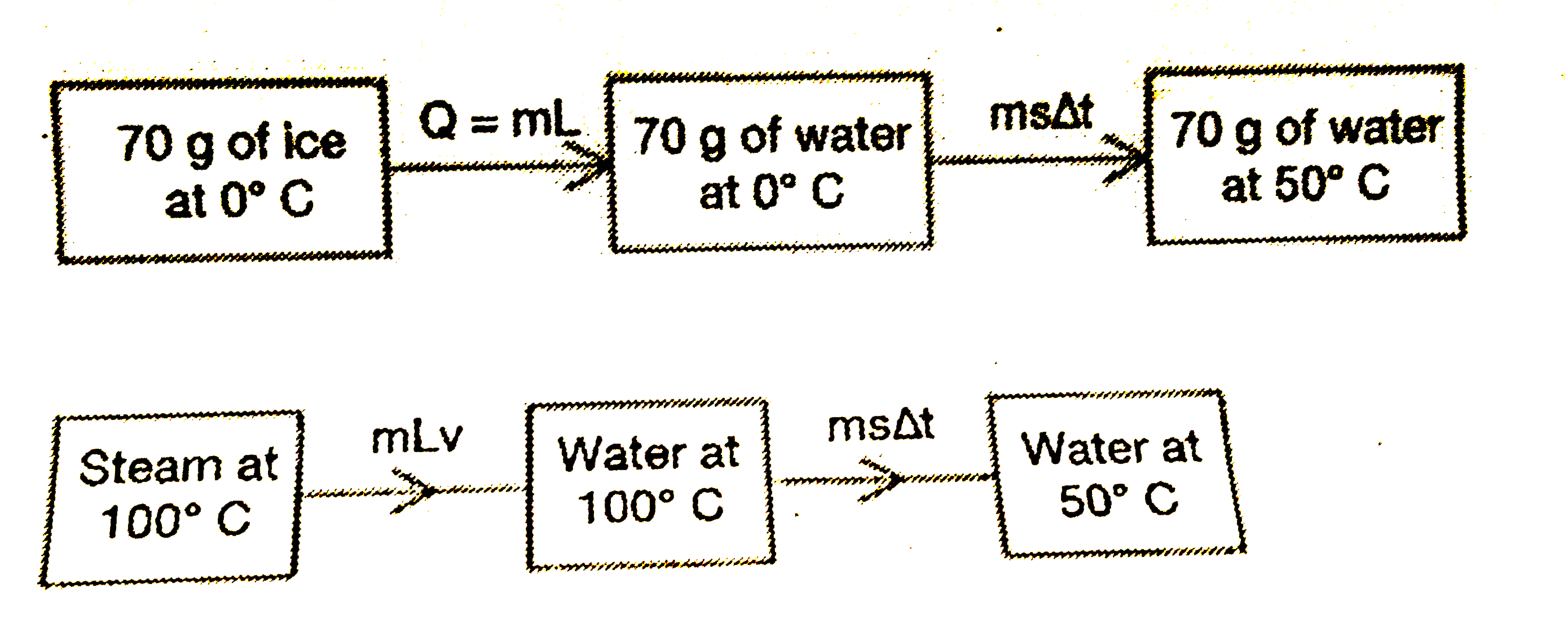
- To obtain of water at 50^(@)C, how many grams of stea at its stea poin...
Text Solution
|