Text Solution
Verified by Experts
Topper's Solved these Questions
LANGUAGE OF CHEMISTRY AND CHANGES AROUND US
PEARSON IIT JEE FOUNDATION|Exercise Crossword|2 VideosLANGUAGE OF CHEMISTRY AND CHANGES AROUND US
PEARSON IIT JEE FOUNDATION|Exercise TEST YOUR CONCEPTS (VERY SHORT ANSWER TYPE QUESTIONS) FILL IN THE BLANKS|13 VideosLANGUAGE OF CHEMISTRY AND CHANGES AROUND US
PEARSON IIT JEE FOUNDATION|Exercise Concept Application|10 VideosAMAZING AIR
PEARSON IIT JEE FOUNDATION|Exercise Crossword|1 VideosMYSTERY OF MATTER
PEARSON IIT JEE FOUNDATION|Exercise CROSSWORD|1 Videos
Similar Questions
Explore conceptually related problems
PEARSON IIT JEE FOUNDATION-LANGUAGE OF CHEMISTRY AND CHANGES AROUND US -Assessment test
- Name the elements with the following symbols. (a) C (b) N (c ) S...
Text Solution
|
- Complete the following table
Text Solution
|
- Match the followign based on the number of atoms present in one mol...
Text Solution
|
- Write the formulae of compounds with the following information an...
Text Solution
|
- Find the odd one out among the following sets give reasons.
Text Solution
|
- Identify true or false statements among the following and rewrite t...
Text Solution
|
- Identify the correct statements from the following
Text Solution
|
- Identify true or false statements among the following
Text Solution
|
- Are all chemical changes desirable changes ? Justify with at least...
Text Solution
|
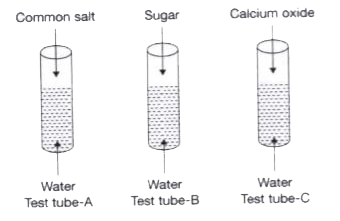
- (a) Identify the test tube(s) in which a chemical change take place. ...
Text Solution
|
- Identify the physical and chemical changes among the following
Text Solution
|
- In which among the following changes, does the molecular composition r...
Text Solution
|
- Analyse the observation of the above experiments
Text Solution
|
- Classify the following into different changes. (a) Making of dough f...
Text Solution
|
- Study the diagrams and make a comment on the type of changes.
Text Solution
|