(i) If we do not consider `[H_(3)O^(+)]` from the ionisation of `H_(2)O` then `[H_(3) O^(+)]=[HCI]` i.e., pH=7 which is a pH of a neutral solution .We know that HCI solution is acidic this case the concentration of the acid is very low `(10^(-7) M)`. Hence the `H_(3) O^(+)(10^(-7 M)`
So in this case we should ionisation of water cannot be neglected.
(ii) The redox process which causes the deterioration of metal is called corrosion Rusting of iron is an example of corrosion.It is an electro chemical process.
(b) i.Adsorption isotherms represent the variation of adsorption at constant temperature adsorption isothem can be studied quatitatively
(ii) A plot between the amount of adsorbate adn pressure or concentration of .
(iii) Freundich adsorption isotem.
(iv) this equation is application for adsorption of gases on solid surfaces .The Same equation becomes `(x)/(m)=ke^(1//n)` when used for adsorption in solution with C as concentration
(v ) these equation quantitively predict the effect of pressure (or concentration ) on the adsorption of gases at constant temperature.
(vi) Taking log on both sides of equation
`(x)/(m) =kP^(1//n)`
`log (x )/(m) = log k+ (1)/(n )log P`
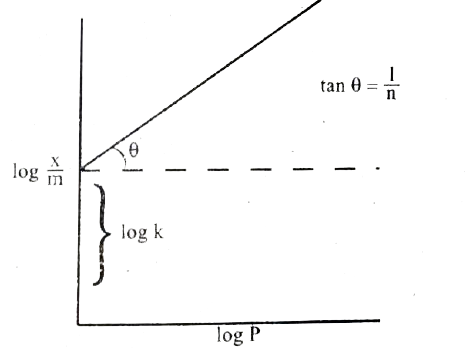
(vii) Hence the intercept represents the value of log k and the slope `(b)/(q)` gives `(1)/(n)`
(vii) this equation explain the increases of `(x)/(m)` with increas in pressure .But experimental values shows the deviation at low pressure .
`(ix) Limitations
(a) This equation is purely empirical over a limited pressure range
