Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-SAMPLE PAPER - 3 -PART-III (Answer the questions)
- Write a note on zeolites.
Text Solution
|
- What is Royal water? Mention its uses.
Text Solution
|
- [Ni(CN)4 ]^(2-) is diamagnetic ,while [NiCl4]^(2) - is paramagnetic...
Text Solution
|
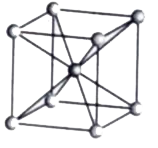
- Calculate the number of atoms per unit cell of bcc type.
Text Solution
|
- Identify the order for the following reactions (i) Rusting of Iron ...
Text Solution
|
- Calculate the (i) hydrolysis constant, (ii) degree of hydrolysis and (...
Text Solution
|
- Write the structure of the aldehyde, carboxylic acid and ester that yi...
Text Solution
|
- Predict the major product that would be obtained on nitration of the f...
Text Solution
|
- What are the biological functions of nucleic acids?
Text Solution
|