Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-SAMPLE PAPER 10 (SOLVED)-Part-III
- Explain the types of silicones.
Text Solution
|
- Give the properties of inter halogen compounds.
Text Solution
|
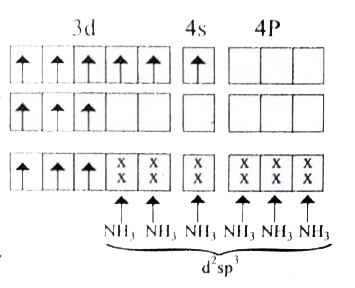
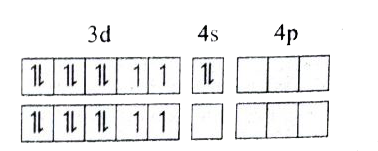
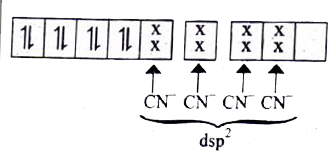
- Based VB theory explain why [Cr(NH3)6]^(3+) is paramagnetic , while [...
Text Solution
|
- A first order reaction is 20% completed in 10 minutes. Calculate the t...
Text Solution
|
- Explain common ion effect with an example.
Text Solution
|
- Mention the uses of Brownian movement.
Text Solution
|
- How will you convert benzaldehyde into the following compounds? (i) ...
Text Solution
|
- Complete the following. (i) CH(3)CN underset(C(2)H(5)OH)overset(Na-H...
Text Solution
|
- Answer the following questions briefly: (i) What are reducing sugars...
Text Solution
|