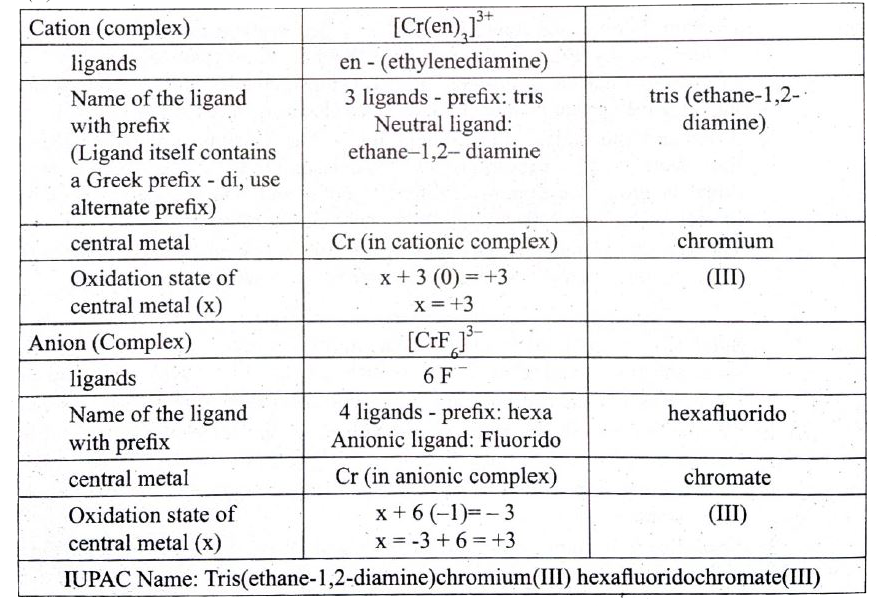
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-SAMPLE PAPER 10 (SOLVED)-Part-IV
- (a) (i) What is Cementation? (ii) Write a notes on ionisation enthal...
Text Solution
|
- (b) (i) Give the uses of sulphuric acid.. (ii) How alloys are formed...
Text Solution
|
- (a) (i) A solution of [Co(NH(3))(4)I(2)]Cl(2) when treated with AgNO(3...
Text Solution
|
- (b) (i) KF crystallizes in fcc structure like sodium chloride. Calcula...
Text Solution
|
- (a) (i) Point out the difference between ionic product and solubility ...
Text Solution
|
- (b) (i) Describe about lithium-ion battery and its uses. (ii) Give r...
Text Solution
|
- (a) (i) Heat of adsorption is greater for chemisorptions than physisor...
Text Solution
|
- (b) (i) How is Aniline converted into Phenol? (ii) What is the actio...
Text Solution
|
- (a) (i) An aromatic compound 'A' of molecular formula C(7)H(7)ON under...
Text Solution
|
- (b) (i) Write a note on co-polymer. (ii) What is the difference betw...
Text Solution
|