Text Solution
Verified by Experts
Topper's Solved these Questions
SAMPLE PAPER - 12 (UNSOLVED)
FULL MARKS|Exercise PART - III|5 VideosSAMPLE PAPER - 12 (UNSOLVED)
FULL MARKS|Exercise PART - IV|4 VideosSAMPLE PAPER - 12 (UNSOLVED)
FULL MARKS|Exercise PART - IV|4 VideosSAMPLE PAPER - 11 (UNSOLVED)
FULL MARKS|Exercise PART - IV|3 VideosSAMPLE PAPER - 13
FULL MARKS|Exercise PART - II|9 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-SAMPLE PAPER - 12 (UNSOLVED)-PART - II
- Mention the uses of Gold (Au).
Text Solution
|
- Why fluorine is more reactive than other halogens?
Text Solution
|
- What is meant by coordination polyhedron?
Text Solution
|
- Write the rate expression for the following reactions, assuming them a...
Text Solution
|
- The concentration of hydroxide ion in a water sample is found to be 2....
Text Solution
|
- Mention Freundlinch adsorption isothermal equation.
Text Solution
|
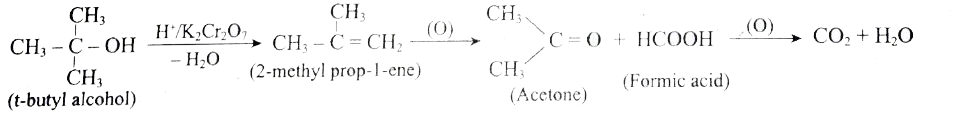
- Is it possible to oxidise t-butyl alcohol using acidified dichromate t...
Text Solution
|
- Explain the action of Diisobutyl aluminium hydride (DIBAL-H) and H(2)O...
Text Solution
|
- Define enzymes.
Text Solution
|