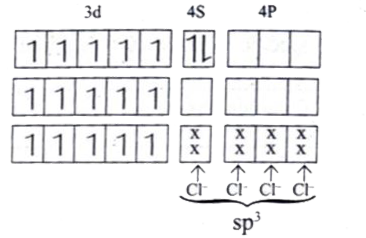
Text Solution
Verified by Experts
Topper's Solved these Questions
SAMPLE PAPER - 16 ( UNSOLVED)
FULL MARKS|Exercise PART - III|7 VideosSAMPLE PAPER - 16 ( UNSOLVED)
FULL MARKS|Exercise PART - IV|5 VideosSAMPLE PAPER - 16 ( UNSOLVED)
FULL MARKS|Exercise PART - IV|5 VideosSAMPLE PAPER - 15 (UNSOLVED )
FULL MARKS|Exercise PART - IV|6 VideosSAMPLE PAPER - 17 (UNSOLVED)
FULL MARKS|Exercise PART - IV|2 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-SAMPLE PAPER - 16 ( UNSOLVED) -PART - II
- What is catenation? Describe briefly the catenation property of carbon...
Text Solution
|
- Which catalyst is used in the hydroformylation of olefins? Give equat...
Text Solution
|
- The spin only magnetic moments of Tetrachloridomanganate (II) ion is 5...
Text Solution
|
- Define order of a chemical reaction.
Text Solution
|
- Calculate the concentration of OH^(-) in a fruit juice which contains ...
Text Solution
|
- Comment on the statement : Colloid is not a substance but it is a stat...
Text Solution
|
- Suggest a reason for the large difference in the boiling point of buta...
Text Solution
|
- Draw the structure of sucrose.
Text Solution
|
- Which sweetening agents are used to prepare sweets for a diabetic pati...
Text Solution
|