A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
FULL MARKS-SAMPLE PAPER - 18 (UNSOLVED)-PART - IV
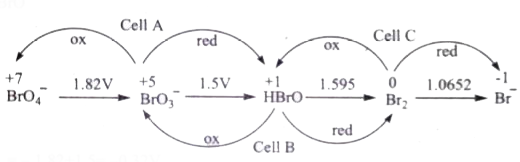
- Consider the change in oxidation state of Bromine corresponding to di...
Text Solution
|
- (a) (i) Explain the following terms with suitable examples. (i) Gangue...
Text Solution
|
- (b) (i) Write the molecular formula and structural formula for the fol...
Text Solution
|
- (a) (i) What are the factors which influence the adsorption of a gas o...
Text Solution
|
- (b) (i) Identify the product(s) is/are formed when 1 - methoxy propane...
Text Solution
|
- (b) Explain free radical polymerisation with example.
Text Solution
|