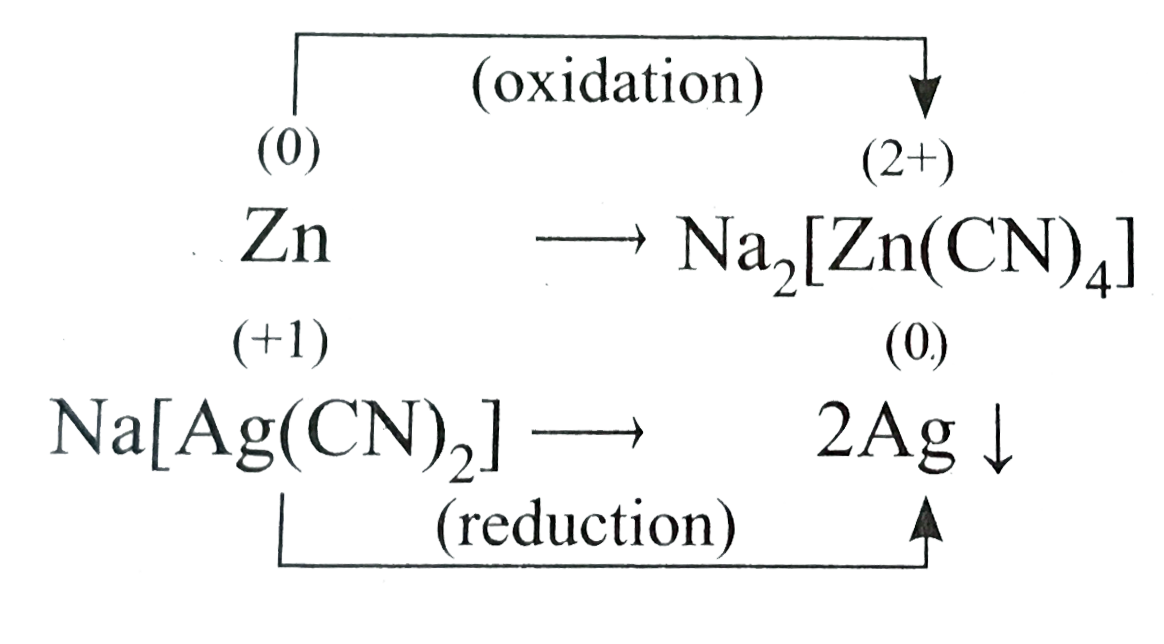
Text Solution
Verified by Experts
Topper's Solved these Questions
METALLURGY
FULL MARKS|Exercise Additional questions ( CHOOSE THE BEST ANSWER )|49 VideosMETALLURGY
FULL MARKS|Exercise Additional questions -Fill in the blanks|28 VideosMETALLURGY
FULL MARKS|Exercise Textbook evaluation -Answer the following questions:|17 VideosIONIC EQUILIBRIUM
FULL MARKS|Exercise ADDITIONAL QUESTIONS (5 MARK QUESTION)|15 VideosORGANIC NITROGEN COMPOUNDS
FULL MARKS|Exercise ADDITIONAL QUESTIONS (5-MARK QUESTIONS)|14 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-METALLURGY -Evaluate Yourself
- Write the equation for the extraction of silver by leaching with sodiu...
Text Solution
|
- Magnesite (Magnesium carbonate) is calcined to obtain magnesia, which ...
Text Solution
|
- using Ellingham diagram indicate the lowest temprature at which ZnO ca...
Text Solution
|
- Metallic sodium is extracted by the electrolysis of brine (eq. NaCl) ....
Text Solution
|