Text Solution
Verified by Experts
Topper's Solved these Questions
p-BLOCK ELEMENTS -I
FULL MARKS|Exercise EVALUATE YOURSELF|1 Videosp-BLOCK ELEMENTS -I
FULL MARKS|Exercise Additional Questions ( CHOOSE THE CORRECT ANSWER ):|46 Videosp-BLOCK ELEMENTS -I
FULL MARKS|Exercise Additional Questions - 5- MARKS QEUSTIONS|10 VideosP - BLOCK ELEMENTS - II
FULL MARKS|Exercise Additional Questions (5 Mark Questions)|11 VideosSAMPLE PAPER - 11 (UNSOLVED)
FULL MARKS|Exercise PART - IV|3 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-p-BLOCK ELEMENTS -I-TEXT BOOK EVALUATION- ANSWER THE FOLLOWING QUESTIONS:
- Write the short note on anamolous properties of the first element of p...
Text Solution
|
- (a) Describe briefly allotropism in p - block elements with specific r...
Text Solution
|
- Boron does not react directly with hydrogen. Suggest one method to pre...
Text Solution
|
- Give the uses of Borax.
Text Solution
|
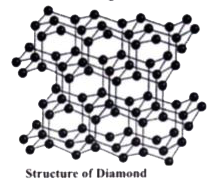
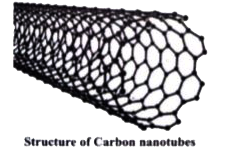
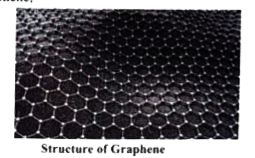
- What is catenation? Describe briefly the catenation property of carbon...
Text Solution
|
- Write a note on Fisher tropsch synthesis.
Text Solution
|
- Give the structure of CO and CO(2)
Text Solution
|
- Give the uses of silicones.
Text Solution
|
- AlCl(3) behaves like a lewis acid. Substantiate this statement.
Text Solution
|
- Describe the structure of diborane .
Text Solution
|
- Write a short note on hydroboration.
Text Solution
|
- Give one example for each of the following (i) icosogens (ii) ...
Text Solution
|
- Write a note on metallic nature of p-block elements.
Text Solution
|
- Complete the following reactions: a. B(OH)(3)+NH(3)to (b) Na(2)B(4)O...
Text Solution
|
- How will you identify borate radical ?
Text Solution
|
- Write a note on zeolites.
Text Solution
|
- How will you convert boric acid to boron nitride ?
Text Solution
|
- A hydride of 2^(nd) period alkali metal (A ) on reaction with c...
Text Solution
|
- A double salt which contains fourth period alkali metal (A) on heating...
Text Solution
|
- CO is a reducing agent. Justify with an example.
Text Solution
|