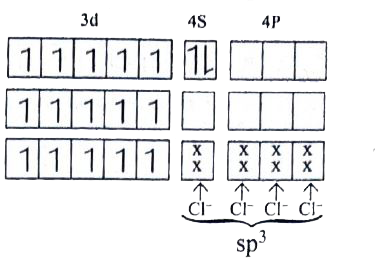
Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION CHEMISTRY
FULL MARKS|Exercise Textbook Evaluation|50 VideosCOORDINATION CHEMISTRY
FULL MARKS|Exercise Additional Question(Choose the correct answer)|76 VideosCHEMISTRY IN EVERYDAY LIFE
FULL MARKS|Exercise ADDITIONAL QUESTION ( 5 Mark Questions )|2 VideosELECTRO CHEMISTRY
FULL MARKS|Exercise ADDITIONAL QUESTIONS -5- MARK QUESTIONS|15 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-COORDINATION CHEMISTRY-Additional Question(5 Marks Question)
- The spin only magnetic moment of Tetrachloridomaganate (II) ion is 5.9...
Text Solution
|
- Write the IUPAC name of the following complexes. (i) [Ag(NH(3))(2)]C...
Text Solution
|
- Explain about the geometrical isomerism in complexes having coordinati...
Text Solution
|
- Explain about the geometrical isomerism of octahedral complexes with s...
Text Solution
|
- Describe about the postulate of VB theory (or) Valence bond theory.
Text Solution
|
- Explain the hybridisation, mgnetic property, geometry, magnetic moment...
Text Solution
|
- Explain the hybridisation, geometry, magentic property and magnetic mo...
Text Solution
|
- Using VB theory, explain the type of hybridisation , geometry , magnet...
Text Solution
|
- Explain the hybridisation, geometry, magentic property and magnetic mo...
Text Solution
|
- Explain about crystal field theory.
Text Solution
|
- Describe about the crystal field splitting in tetrahedral complex.
Text Solution
|
- How would you calculate crystal field stabilization energy (CBSE) for ...
Text Solution
|
- How would you measure CBSE for [Fe(CN)(6)]^(3-).
Text Solution
|
- Explain about the classification of metal carbonyls with suitable exam...
Text Solution
|
- Explain about the importance and application of coordination complexes...
Text Solution
|
- Write the formulae for the following coordination compounds : (i) Te...
Text Solution
|
- Write the IUPAC names of the following (i)[Co(NH(3))(6)Cl(3) (ii)[Co...
Text Solution
|
- Indicate the types of isomerism exhibited by the following complexes a...
Text Solution
|
- Give evidence that [Co(NH(3))(5)Cl]SO(4) and [CO(NH(3))(5)SO(4)]Cl are...
Text Solution
|
- Explain on the basis of valence bondy theory that [Ni(CN)(4)]^(2-) ion...
Text Solution
|
- [NiCl(4)]^(2-) is paramagnetic while [Ni(CO)(4)] is diamagnetic though...
Text Solution
|