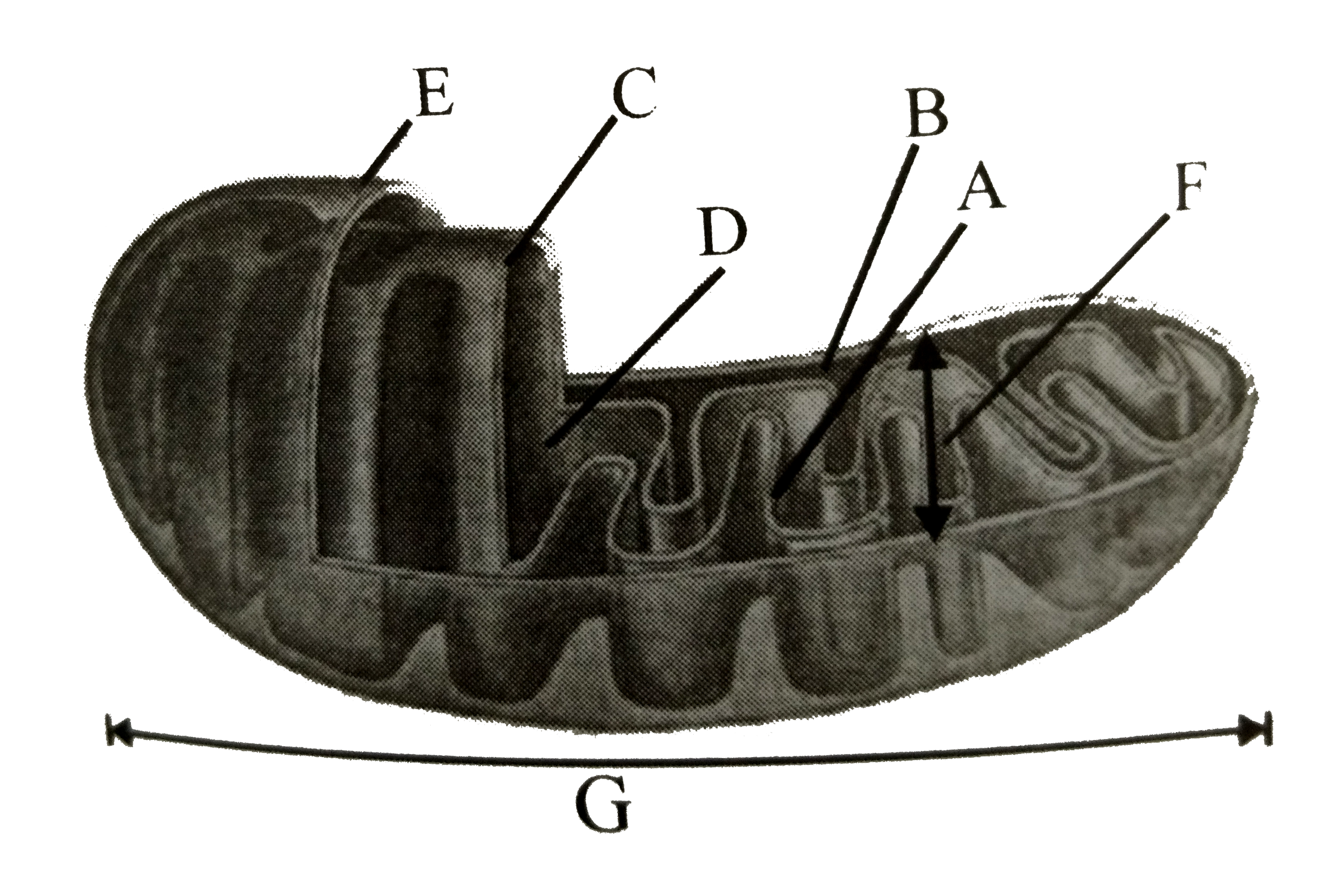
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which rpresents aqueous compartments ?
Text Solution
|
- Consider a wave rpresented by y= a cos^(2) (omega t-kx) where symbols...
Text Solution
|
- In a stationary railway compartment, there are several passengers. If ...
Text Solution
|
- The total lung capacity is rpresented by
Text Solution
|
- In the male reproductive system of cockroach A, B, C, D and E r...
Text Solution
|
- Which is not a compartment in the cell ?
Text Solution
|
- The middle compartment of the hydrogen-oxygen fuel cell contains, hot ...
Text Solution
|
- Assertion :- Mitochondrial lumen is distinctly divided into two aqueou...
Text Solution
|
- There are 10 compartments in passenger train which carries on an avera...
Text Solution
|