Text Solution
Verified by Experts
Topper's Solved these Questions
BASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise ILLUSTRATIONS OF OBJECTIVE QUESTIONS|20 VideosBASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise PROBLEMS FOR PRACTICE|17 VideosBASIC PRINCIPLES
OP TANDON|Exercise SECTION V INTEGER ANSWER TYPE QUESTION|3 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-BASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)-SINGLE INTEGER ANSWER TYPE QUESTIONS
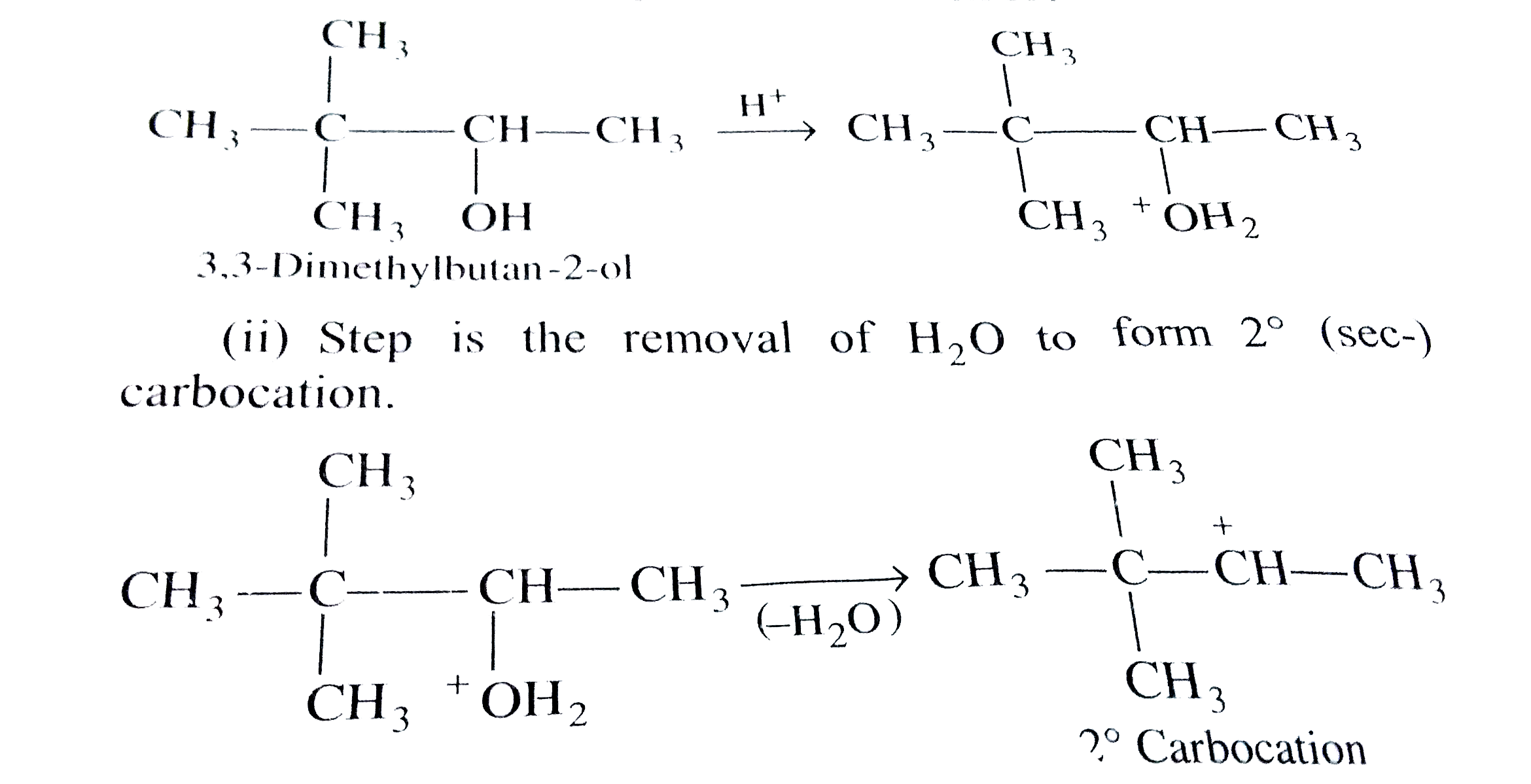
- 3,3-dimethylbutan-2-ol losses a molecule of water in the presence of c...
Text Solution
|
- Amongst following the total number of electrophiles is: Cl^(+),OH^(-...
Text Solution
|
- Amongst following the total number of nucleophiles is: R^(-),OR^(-),...
Text Solution
|
- The total number of contributing structures showing hyperconjugation (...
Text Solution
|
- Total number of Lewis acids among the following is : BF(3),H(3)PO(4)...
Text Solution
|