A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise OBJECTIVE QUESTIONS (Level-B)|25 VideosBASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise SET II|24 VideosBASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise BRAIN STORMING PROBLEMS|14 VideosBASIC PRINCIPLES
OP TANDON|Exercise SECTION V INTEGER ANSWER TYPE QUESTION|3 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-BASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)-OBJECTIVE QUESTIONS (Level-A)
- The hydrolysis of 2-bromo-3-methylbutane by S(N^(1)) mechanism gives m...
Text Solution
|
- The electrophile, E^((o+)) attacks the benzene ring to generate the in...
Text Solution
|
- Which one of the following carbanions is the least stable?
Text Solution
|
- CH(3)CH(2)Cl undergoes homolytic fission produces:
Text Solution
|
- Tertiary butyl chloride preferably undergo hydrolysis by:
Text Solution
|
- In a S(N^(2)) substitution reaction of the type R-Br+Cl^(-)overset("...
Text Solution
|
- The correct stability order for the following speceis is :
Text Solution
|
- Arrange the carbanions, (CH(3))(3)bar(C),bar(C)Cl(3),(CH(3))(2)bar(C)H...
Text Solution
|
- Which of the following carbocations will be more stable?
Text Solution
|
- Arrange the following free radicals in order of decreasing stability: ...
Text Solution
|
- The most easily hydrolysed molecule under S(N^(1)) condition is:
Text Solution
|
- Least active electrophile is :
Text Solution
|
- In the following carbocation, H//CH(3) that is most likely to migrate ...
Text Solution
|
- Arrange the following resonating structures in order of increasing sta...
Text Solution
|
- Identify a species which is not a Bronsted acid but a Lewis acid:
Text Solution
|
- A solution of (-l)- chloro -1 phenyletane in toluene recemises slowly ...
Text Solution
|
- The order of stability of the following carbocations: underset("I")(...
Text Solution
|
- The hyperconjugative stabilities of tert-butyl cation and 2-butene, re...
Text Solution
|
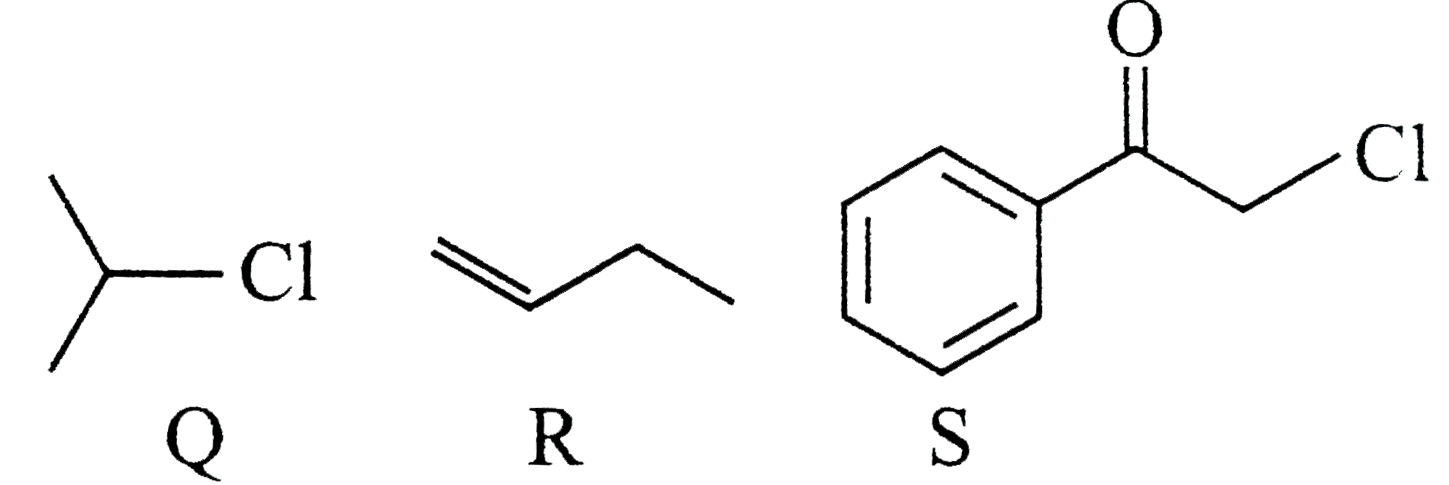
- KI in acetone, undergoes S(N)2 reaction with each of P,Q ,R and S The ...
Text Solution
|
- Which of the following molecules is least resonance stabilised?
Text Solution
|
 .
.