A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise SET II|24 VideosBASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise ASSERTION-REASON TYPE QUESTIONS|15 VideosBASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise OBJECTIVE QUESTIONS (Level-A)|193 VideosBASIC PRINCIPLES
OP TANDON|Exercise SECTION V INTEGER ANSWER TYPE QUESTION|3 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-BASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)-OBJECTIVE QUESTIONS (Level-B)
- Electrophile NO(2)^(+) attacks on the following: In which cases w...
Text Solution
|
- Dehydrobromination (-HBr) of the following in increasing order will be...
Text Solution
|
- The above trasformation proceeds through:
Text Solution
|
- Maximum stability will be in which of the following free radicals?
Text Solution
|
- Which of the following structures correspond to the product expected, ...
Text Solution
|
- The intermediate during the addition of HCl to propene in the presence...
Text Solution
|
- A covalently strated host group present in the benzene nucleus is orth...
Text Solution
|
- Select the incorrect statement among the following
Text Solution
|
- Kharasch effect regarding addition of HBr is not observed in:
Text Solution
|
- Among the following the aromatic compound is
Text Solution
|
- Identify the correct order of reactivity in electrophilic substitution...
Text Solution
|
- In which of the following, first memeber is more stable than second?
Text Solution
|
- Most stable carbocation is:
Text Solution
|
- Which of the following is least stable ?
Text Solution
|
- The structure of Wheland intermediate obtained after the attack of Br^...
Text Solution
|
- Which of the following halides will be most reactive towards S(N^(2)) ...
Text Solution
|
- Conjugation of electron withdrawing groups, e.g., -CHO, -overset(O)o...
Text Solution
|
- Relative stabilities of the following carbocations will be in the orde...
Text Solution
|
- Consider the reaction : CH(3)CH(2)CH(2)Br+NaCNrarrCH(3)CH(2)CH(2)CN+...
Text Solution
|
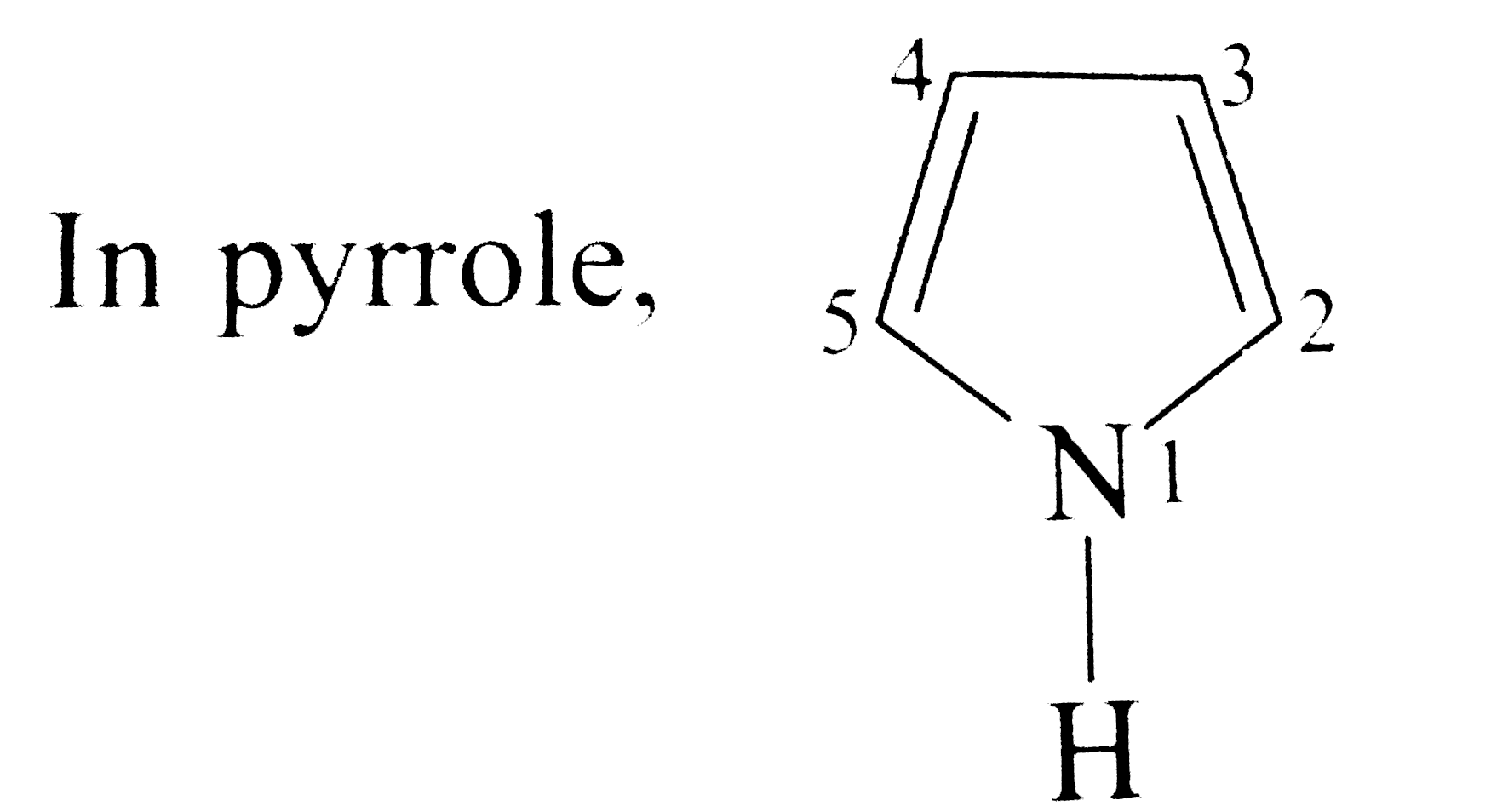
- In pyrrole, The electron density is maximum on :
Text Solution
|