A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
RADIOACTIVITY AND NUCLEAR TRANSFORMATION
OP TANDON|Exercise SECTION - IV|2 VideosRADIOACTIVITY AND NUCLEAR TRANSFORMATION
OP TANDON|Exercise SECTION - V|3 VideosRADIOACTIVITY AND NUCLEAR TRANSFORMATION
OP TANDON|Exercise SECTION - II|5 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OP TANDON|Exercise Single integer|8 VideosSATURATED ALIPHATIC HYDROCARBONS
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|7 Videos
OP TANDON-RADIOACTIVITY AND NUCLEAR TRANSFORMATION -SECTION - III
- Statement-2 beta particle are emitted by nucleus Because Statement...
Text Solution
|
- Statement-1 : Phosphours-32 decays to sulphur-32 with emission of a be...
Text Solution
|
- Statement-1 : Energy is released in the nuclear fusion of hydrogen nuc...
Text Solution
|
- Statement-1 : .(56)^(133)Ba + e^(-) to .(55)^(133)Cs + X-ray Because...
Text Solution
|
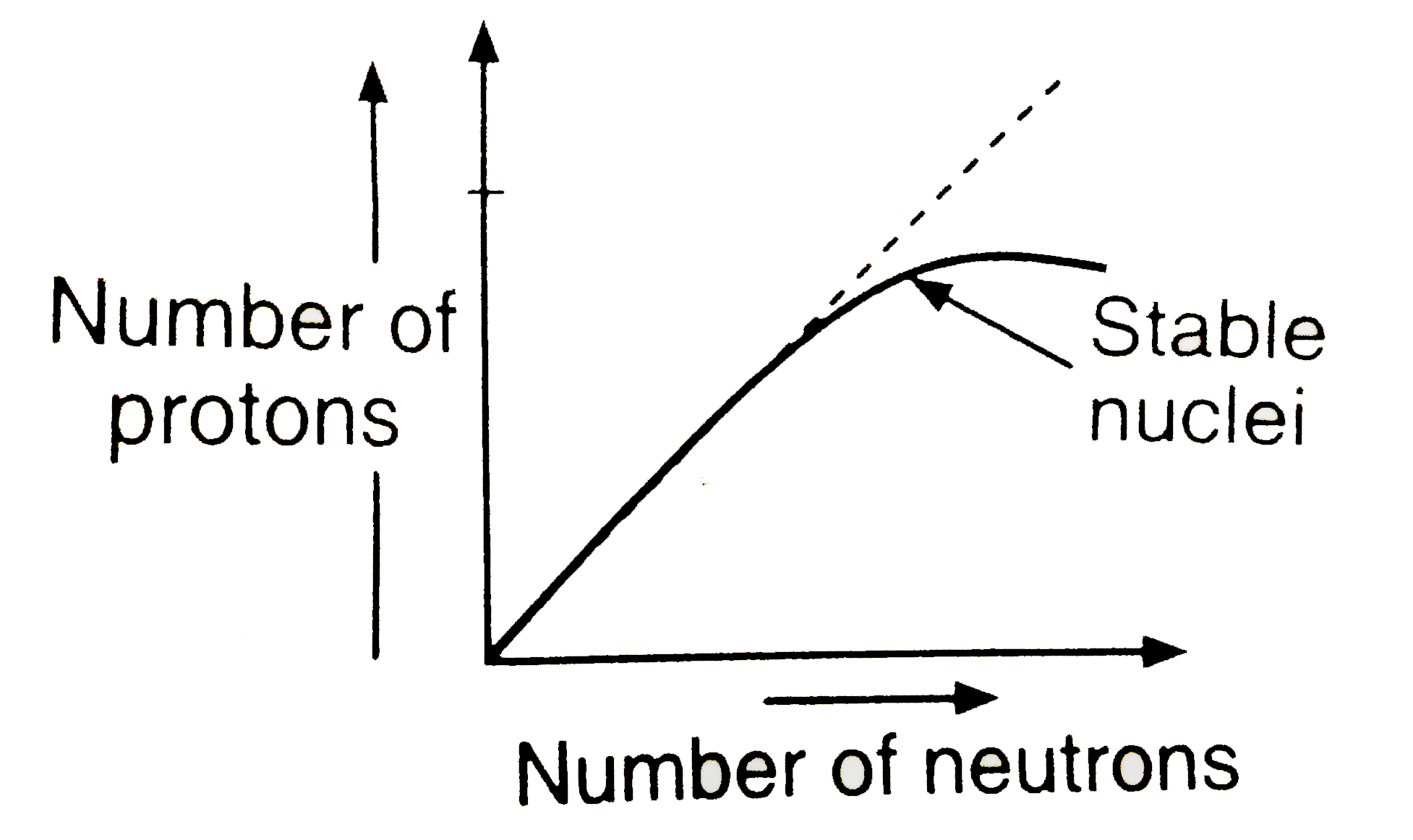
- Statement : The plot of atomic number ( y -axis ) versus number of neu...
Text Solution
|