A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-KARNATAKA CET 2001-Chemistry
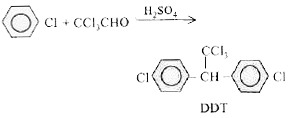
- To get DDT chlorobenzene has to react with the following compound in t...
Text Solution
|
- Alcoholic potash is used to bring about
Text Solution
|
- Propanone is the product obtained by dehydrogenation of
Text Solution
|
- Phenol is treated which bromine water and shaken well. The white preci...
Text Solution
|
- Which of the following organic compounds answers both Iodoform test an...
Text Solution
|
- The quantify of electricity required to liberate 112 cm^(3) of hydroge...
Text Solution
|
- Which one of the following can be classified as Bronsted base?
Text Solution
|
- Hydrogen ion concentration of an aqueous solution is 1 xx 10^(-4)M. Th...
Text Solution
|
- pKa values of two acids A and B are 4 and 5. The strengths of these tw...
Text Solution
|
- pH of a solution produced when an aqueous solution of pH 6 is mixed wi...
Text Solution
|
- IUPAC name of K(3)[Fe(CN)(6)] is
Text Solution
|
- The shape of cuprammonium ion is
Text Solution
|
- When a mixture of calcium acetate and calcium formate is dry distilled...
Text Solution
|
- The test used for identifying peptide linkage in protein is
Text Solution
|
- Which of the following compounds on boiling with alkaline KMnO(4) and ...
Text Solution
|
- 5 moles of SO(2) and 5 moles of O(2) are allowed to react to form SO(3...
Text Solution
|
- A quantity of PCl(5) was heated in a 10 dm^(3) vessel at 250^(@)C PC...
Text Solution
|
- For the reaction H(2)O((s)) hArr H(2)O((l)) at ""^(@)C and normal pres...
Text Solution
|
- In which of the following equilibrium system is the rate of the backwa...
Text Solution
|
- The conversion of A to B follows second order kinetics. Doubling the c...
Text Solution
|