A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-KARNATAKA CET 2007-CHEMISTRY
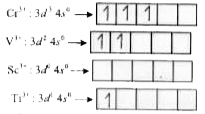
- The electronic configuration of Cr^(3+) is
Text Solution
|
- The mass of metal, with equivalent mass 31.75 which would combine with...
Text Solution
|
- Which of the following forms a colourless solution in aqueous medium?
Text Solution
|
- When a sulphur sol is evaporated sulphur is obtained.On mixing with wa...
Text Solution
|
- An alkyl halide reacts with alcoholic ammonia in a sealed tube, the pr...
Text Solution
|
- When concentrated H(2)SO(4) is heated with P(2)O(5), the acid is conve...
Text Solution
|
- Entropy of the universe is
Text Solution
|
- Which of the following salts on being dissolved in water gives pH gt 7...
Text Solution
|
- The reagent used in Clemmensen reduction is
Text Solution
|
- When KBr is dissolved in water. K^(+) ions are
Text Solution
|
- The noble gas mixture is cooled in a coconut bulb at 173 K.The gases t...
Text Solution
|
- The volume of 10 N and 4 N HCl required to make 1 L of 7 N HCl are
Text Solution
|
- A metal present in insulin is
Text Solution
|
- Carbon forms two oxides which have different compositions.The equivale...
Text Solution
|
- Maximum number of molecules of CH(3)I that can react with a molecule o...
Text Solution
|
- Ellingham diagram represents a graph of
Text Solution
|
- Identify the ore not containing iron.
Text Solution
|
- In which of the following process, a maximum increase in entropy is ob...
Text Solution
|
- Decomposition of benzene diazonium chloride by using (Cu(2)CI(2))/(HCI...
Text Solution
|
- Which complex cannot ionise in solution?
Text Solution
|