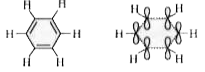A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-KARNATAKA CET 2008-CHEMISTRY
- The number of nodel planes present in sigma^(*)s antibonding orbitals...
Text Solution
|
- Which of the following electrolytic solutionls has the least specific ...
Text Solution
|
- The overlapping of orbitals in benzene is of the type
Text Solution
|
- The calculated bond order in superoxide ion is :
Text Solution
|
- Which of the following can be measured by the Ostwald - Walker dynamic...
Text Solution
|
- Three moles of PCl(5) , three moles of PCl(3) and two moles of Cl(2) a...
Text Solution
|
- How many optically active stereomers are possible for butane - 2,3- di...
Text Solution
|
- An octahedral complex is formed when hybrid orbitals of the following ...
Text Solution
|
- For the reaction 2HI((g))hArrH(2(g))+I(2(g))-QkJ, , the equilibrium c...
Text Solution
|
- The angle strain in cyclobutanne is
Text Solution
|
- The maximum possible number of hydrogen bonds a water molecule can for...
Text Solution
|
- A gas deviates from ideal behaviour at a high pressure because its mol...
Text Solution
|
- The reagent used to convert an alkyne to alkene is
Text Solution
|
- When compared to DeltaG^(@) for the formation of Al(2)O(3), the DeltaG...
Text Solution
|
- In order to increase the volume of a gas by 10%, the pressure of the g...
Text Solution
|
- n- propyl bromide on treating with alcoholic KOH produces
Text Solution
|
- Mercury is a liquid metal because
Text Solution
|
- A compound is formed by elements A and B . This crystallises in the c...
Text Solution
|
- Anisole can be prepared by the action of methly iodide on sodium phen...
Text Solution
|
- Malleability and ductility of metals can be accounted due to
Text Solution
|