A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-KARNATAKA CET 2012-CHEMISTRY
- The pH of the solution obtained by mixing 100 mL of a solution of pH =...
Text Solution
|
- The equilibrium constant of the reaction : A((s)) + 2 B((aq))^(+) hArr...
Text Solution
|
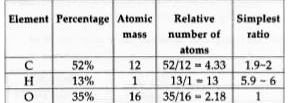
- An oxygen containing organic compound was found to contain 52% carbon ...
Text Solution
|
- Which one of the following is not true regarding electromeric effect ?
Text Solution
|
- Which one of the following is not formed when a mixture of methyl brom...
Text Solution
|
- Power alcohol is a mixture of
Text Solution
|
- Identify 'C' in the following
Text Solution
|
- 20 mL of methane is completely burnt using 50 mL of oxygen . The volum...
Text Solution
|
- 100 mL of 0.1 M acetic acid is completely neutralized using a standard...
Text Solution
|
- Saccharin , an artificial sweetener , is manufactured from
Text Solution
|
- Which of the following is not true for S(N) 1 reaction ?
Text Solution
|
- Oil of winter green is
Text Solution
|
- An organic compound 'A' burns with a sooty flame . It is negative towa...
Text Solution
|
- For a reaction : A to B to Products , the rate of the reaction at va...
Text Solution
|
- Which one of the following has no unpaired electrons ?
Text Solution
|
- The atomic number of cobalt is 27 . The EAN of cobalt in Na(3)[Co(NO(2...
Text Solution
|
- The "spin only" magnetic moment of Ni^(2+) in aqueous solution would b...
Text Solution
|
- Impossible orbital among the following is
Text Solution
|
- The total number of electrons in 18 mL of water (density = 1 g mL^(-1)...
Text Solution
|
- The number of moles of hydrogen that can be added to 1 mole of an oil ...
Text Solution
|