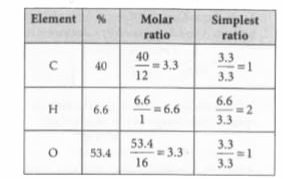
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-KARNATAKA CET 2015-CHEMISTRY
- 0.30g of an organic compound containing C, H and Oxygen on combustion...
Text Solution
|
- For one of the element various successive ionization ethalpies ( in...
Text Solution
|
- The aqueous solution of following salt will have the lowest pH :
Text Solution
|
- One of the following is an essential amino acid.
Text Solution
|
- The formation of cyanohydrin from a ketone is an example of
Text Solution
|
- 100 cm^3 of 1 M CH3COOH was mixed with 100 cm^3 of 2 M CH3OH to form a...
Text Solution
|
- How many coulombs of electricity are required for the oxidation of one...
Text Solution
|
- Cheilosis and digestive disorders are due to the deficiency of
Text Solution
|
- One of the following amide will not undergo Hoffmann bromamide reactio...
Text Solution
|
- Iodoform can be prepared from all, except
Text Solution
|
- The arrangement of following compounds : i. bromomethane ii.brom...
Text Solution
|
- The complex ion having minimum magnitude of Deltao(CFSE) is
Text Solution
|
- Which of the following colloids cannot be easily coagulated ?
Text Solution
|
- After adding non-volatile solute freezing point of water decreases to ...
Text Solution
|
- Which of the following compound of Xenon has pyramidal geometry ?
Text Solution
|
- Cryolite is
Text Solution
|
- Identify 'Q' in the following sequence of reactions :
Text Solution
|
- What ammount of dioxygen (in gram) contains 1.8 xx 10^23 molecules ?
Text Solution
|
- The pair of compound which cannot exist together in solution is
Text Solution
|
- Plot of Maxwell's distribution of velocities is given below : Whi...
Text Solution
|