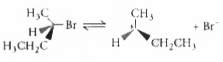
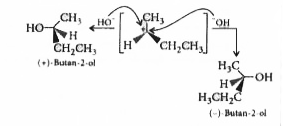
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-KARNATAKA CET 2015-CHEMISTRY
- 10 g of a mixture of BaO and CaO requires 100cm^(3) of 2.5 M HCl to r...
Text Solution
|
- The ratio of heats liberated at 298 K from the combustion of one kg of...
Text Solution
|
- Impure copper containing Fe, Au, Ag as impurities is electrolytically ...
Text Solution
|
- 25 cm^(3) of oxalic acid completely neutralised 0.064 g of sodium hydr...
Text Solution
|
- The statement that is NOT correct is
Text Solution
|
- For the equilibrium: CaCO(3(s)) iff CaO((s)) + CO(2(g)) , Kp = 1.64 at...
Text Solution
|
- Conversion of oxygen into ozone is non-spontaneous at
Text Solution
|
- Density of carbon monoxide is maximum at
Text Solution
|
- The acid strength of active methylene group in : (i) CH(3)COCH(2)COO...
Text Solution
|
- A metallic oxide reacts with water to from its hydroxide, hydrogen per...
Text Solution
|
- X underset("(Reductive)") overset("Ozonolysis")to Y + Z Y can be obt...
Text Solution
|
- Gold sol is not a
Text Solution
|
- Carbocation as an intermediate is likely to be formed in the reaction ...
Text Solution
|
- For an ideal binary liquid mixture
Text Solution
|
- For hydrogen - oxygen fuel cell at one atm and 298 K H(2(g)) + (1)...
Text Solution
|
- The product 'B' is
Text Solution
|
- The correct statement is
Text Solution
|
- P underset(2.H3O^(+))overset(1. CH3MgBr) to R overset(1. dil. NaOH)und...
Text Solution
|
- When CH2 = CH -O - CH2 - CH3 reacts with one mole of HI , one of the ...
Text Solution
|
- 0.44 g of a monohydric alcohol when added to methylmagnesium iodide in...
Text Solution
|