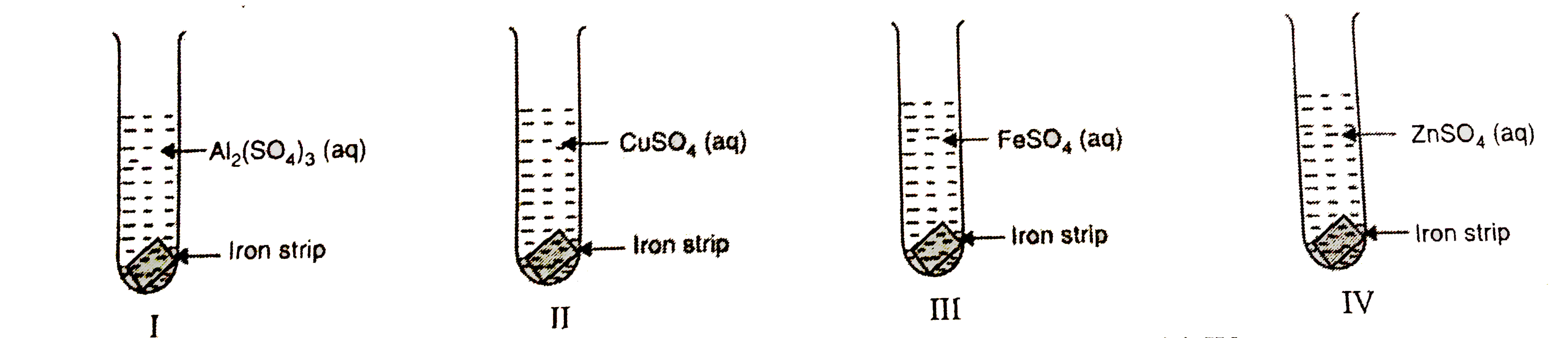Similar Questions
Explore conceptually related problems
Recommended Questions
- A student took four test tubes I,II,III and IV containing aluminium su...
Text Solution
|
- When an aluminium strip is kept immersed in freshly prepared ferrous s...
Text Solution
|
- A student took four test tubes containing solutions of different colou...
Text Solution
|
- A student took four test tubes I,II,III and IV containing aluminium su...
Text Solution
|
- A student addd few pieces of aluminium metal to two test tubes A and B...
Text Solution
|
- A student addd few pieces of aluminium metal to two test tubes A and B...
Text Solution
|
- When a copper strip is kept immersed in free prepared ferrous sulphat...
Text Solution
|
- Apparatus: Copper wire, iron nail, beaker or big test tube, etc. Che...
Text Solution
|
- A student took four test tubes I, II, III and IV containing aluminium ...
Text Solution
|