Similar Questions
Explore conceptually related problems
Recommended Questions
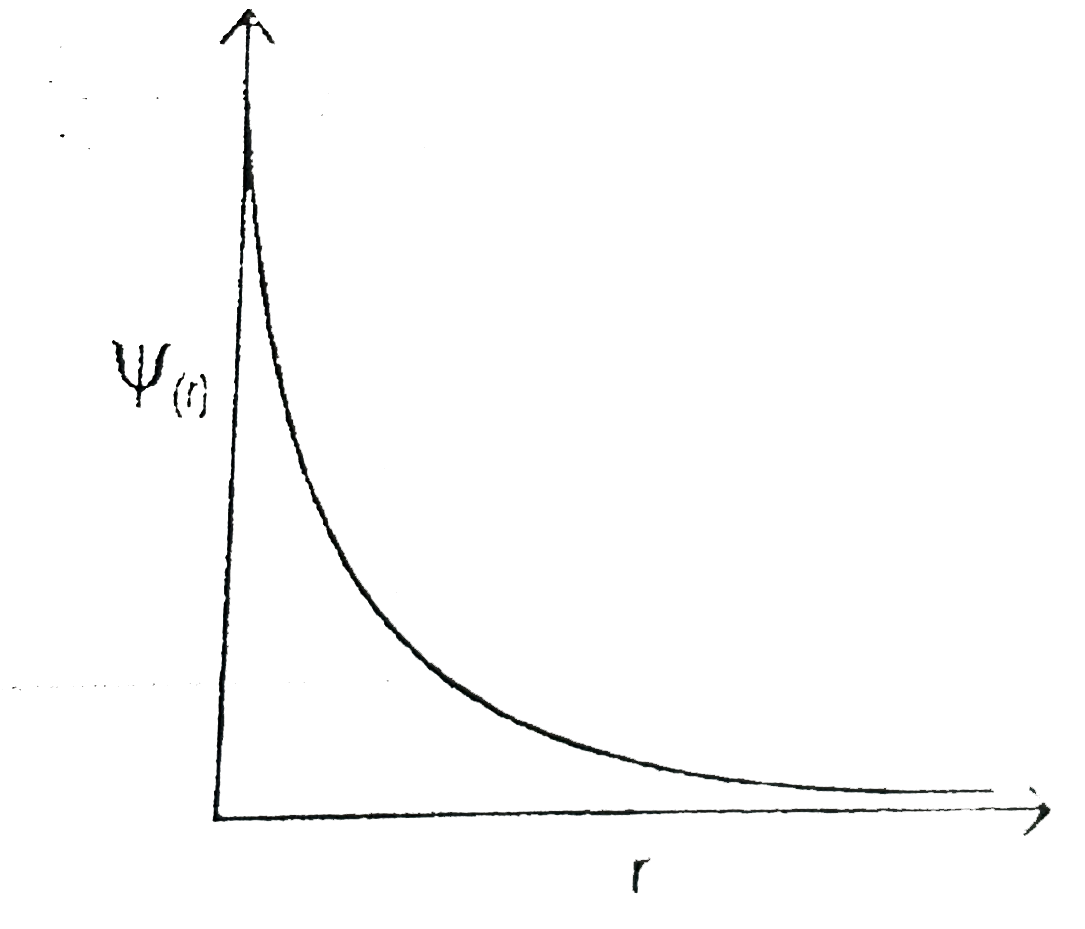
- For an orbital the graph between psi((r )) and r (distance from nucles...
Text Solution
|
- For an orbital the graph between psi((r )) and r (distance from nucles...
Text Solution
|
- For an orbital , having no planar angular nodes following the equation...
Text Solution
|
- Psi((r)) = ke^(-r//k(1)) dot" r^(2) (r^(2) - k(2)r + k(3)) . If the or...
Text Solution
|
- If gravitational forces between a planet and a satellite is proportion...
Text Solution
|
- Two identical satellites are orbiting are orbiting at distances R and ...
Text Solution
|
- The graph that represents the relation between orbital velocity (v(0))...
Text Solution
|
- A planet orbits the Sun in time T at a distance of R from it. Another ...
Text Solution
|
- पथ्वी के केन्द्र से दूरी r पर परिक्रमा करते एक उपग्रह का कक्षीय कोणीय...
Text Solution
|