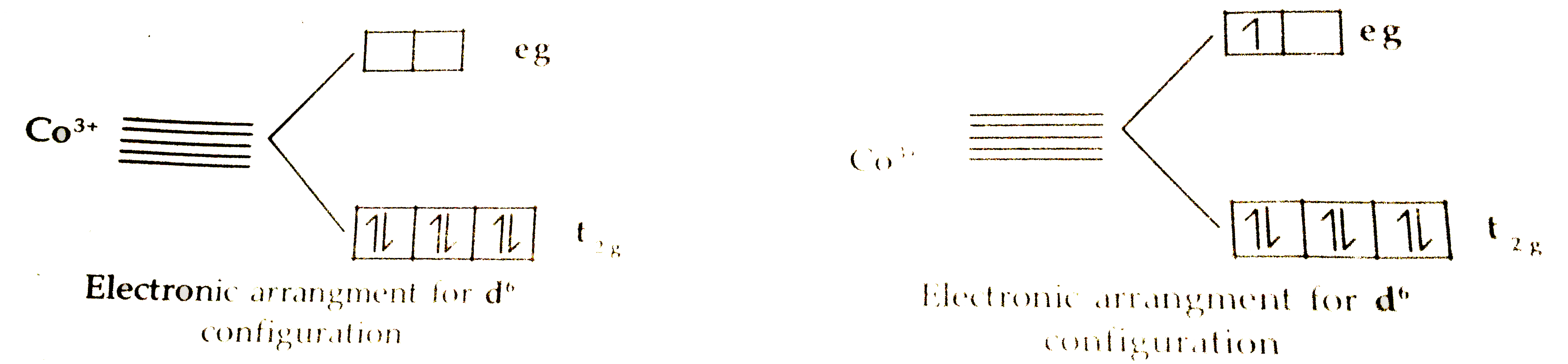
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-DELHI BOARD SET II
- What type of stoichiometric defect is shown by NaCl ? Explain.
Text Solution
|
- Define Emulsions
Text Solution
|
- What role is played by CO(2) in getting pure alumina (Al(2)O(3)) in t...
Text Solution
|
- Draw the structure of 2 bromoentane
Text Solution
|
- Differentiate between molarity and molality of a solution .How can we ...
Text Solution
|
- Assign reason for each of the following : (i) Transition elements ex...
Text Solution
|
- Define the following terms: (i) Sorption (ii) Tyndall effect (ii...
Text Solution
|
- What are following ? Give one example of each (i) Sweetening agents ...
Text Solution
|
- Give names of the monomers of the following polymers: (i) Neoprene ...
Text Solution
|