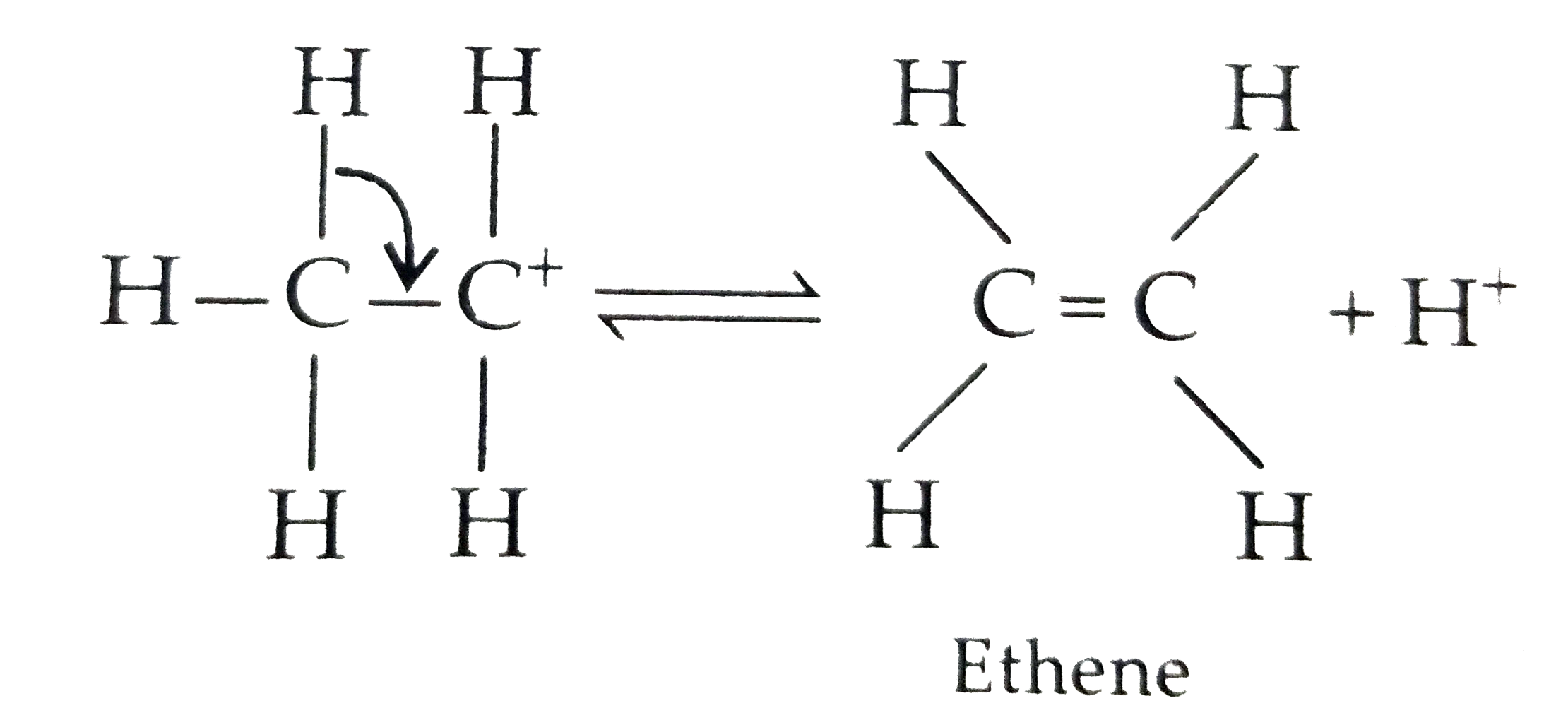
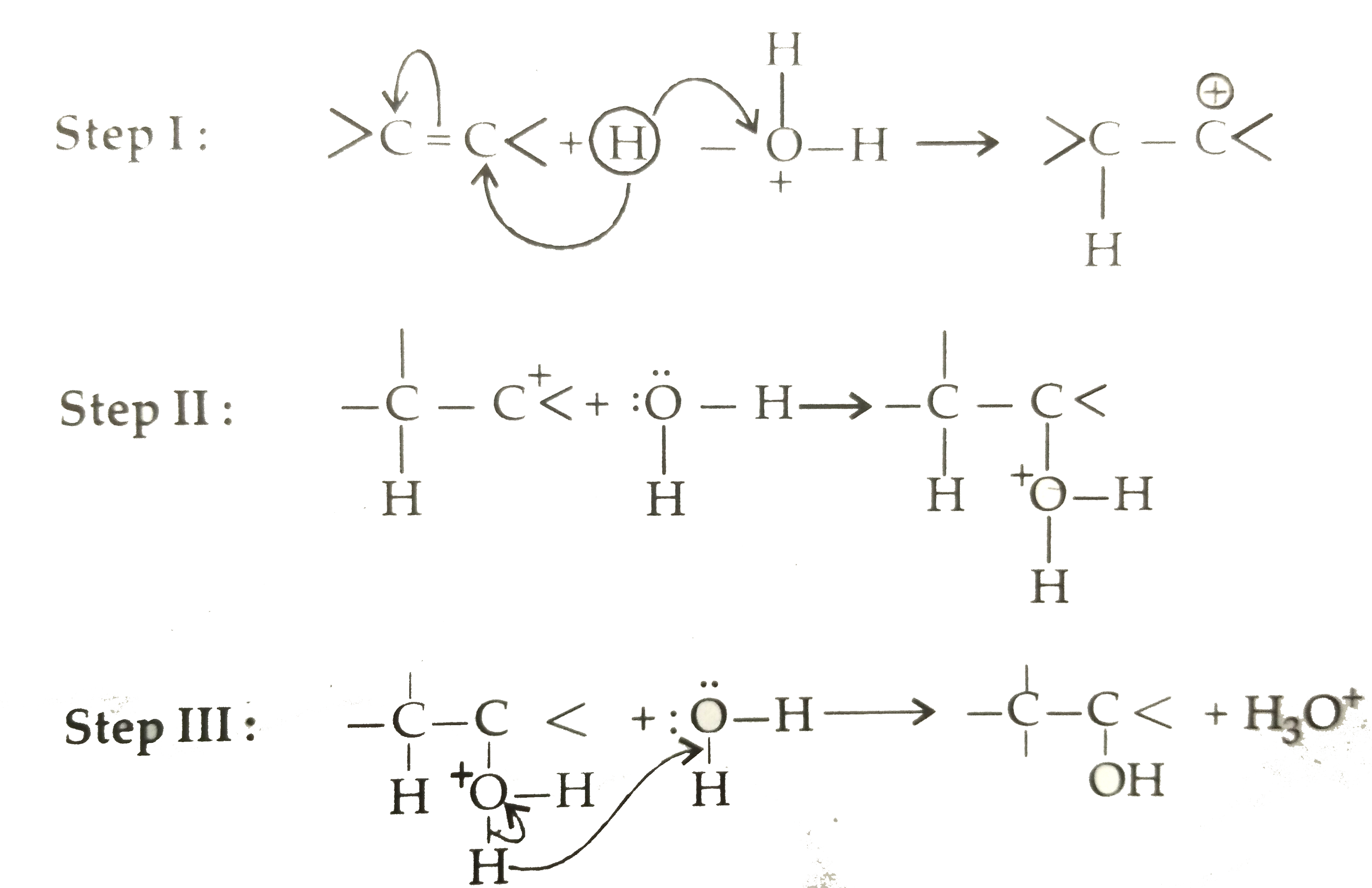
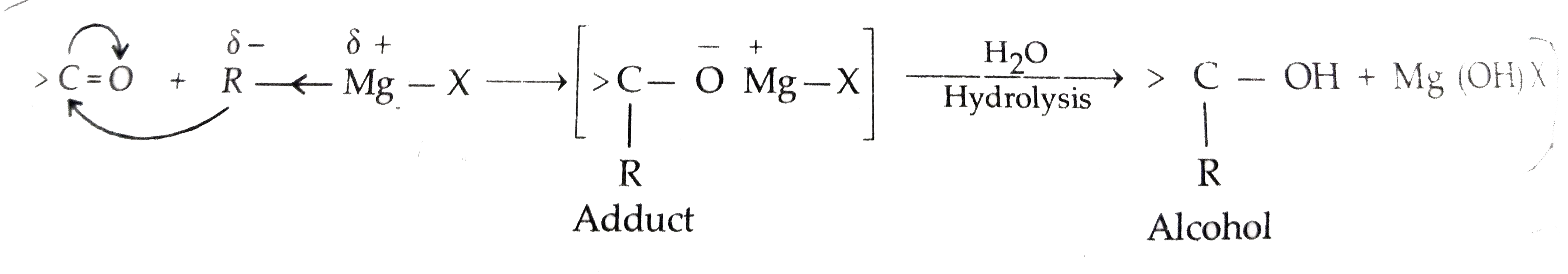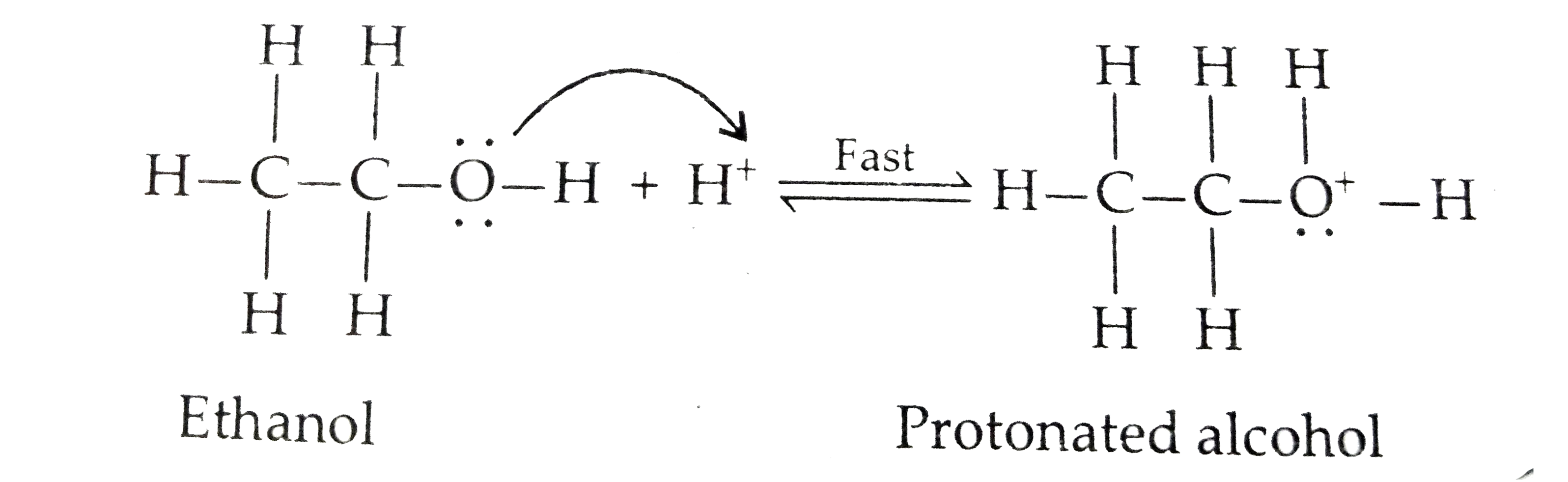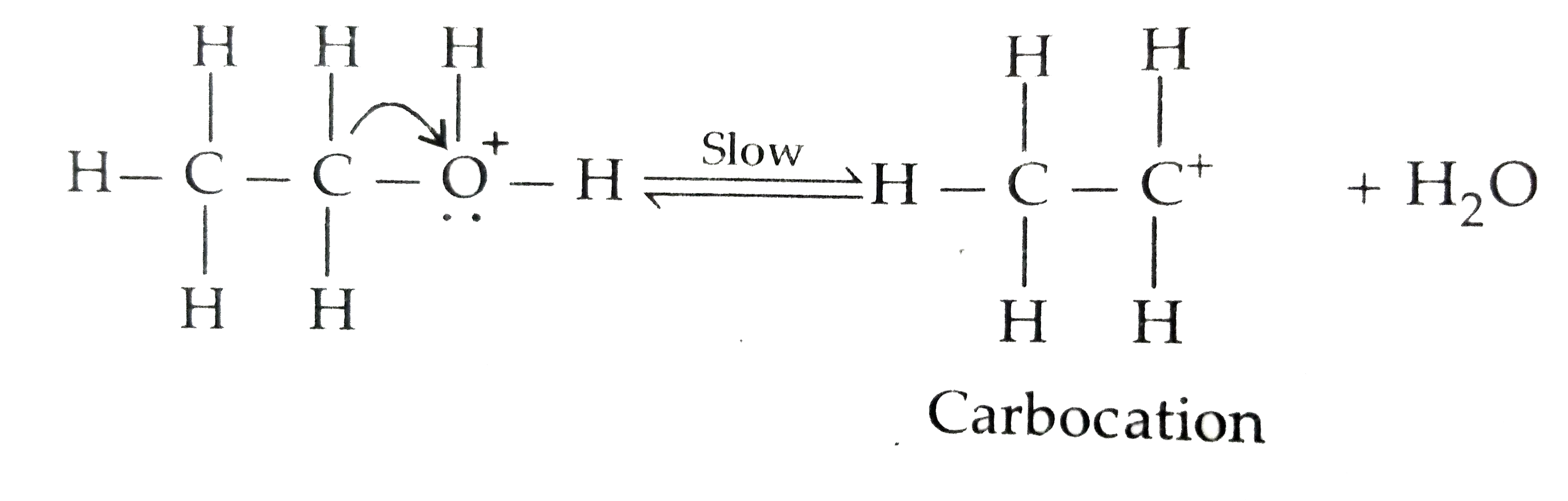Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain the mechanism of the following reactions : (i) Addition of G...
Text Solution
|
- In the acid -catalysed hydration of an alkene , the intermediate forme...
Text Solution
|
- Which of the following alkenes on acid-catalysed hydration gives a ter...
Text Solution
|
- An unsymmetrical alkene in an acid-catalysed hydration could form more...
Text Solution
|
- Explain the mechanism of the following reactions : (i) Addition of G...
Text Solution
|
- Explain the mechanism of acid catalysed of an alkene to form corresp...
Text Solution
|
- Which of the following alkene on acid catalysed hydration form propan-...
Text Solution
|
- Which one of the following alkenes produces a tertiary alcohol on acid...
Text Solution
|
- Explain the mechanism of acid catalysed hydration of an alkene to from...
Text Solution
|