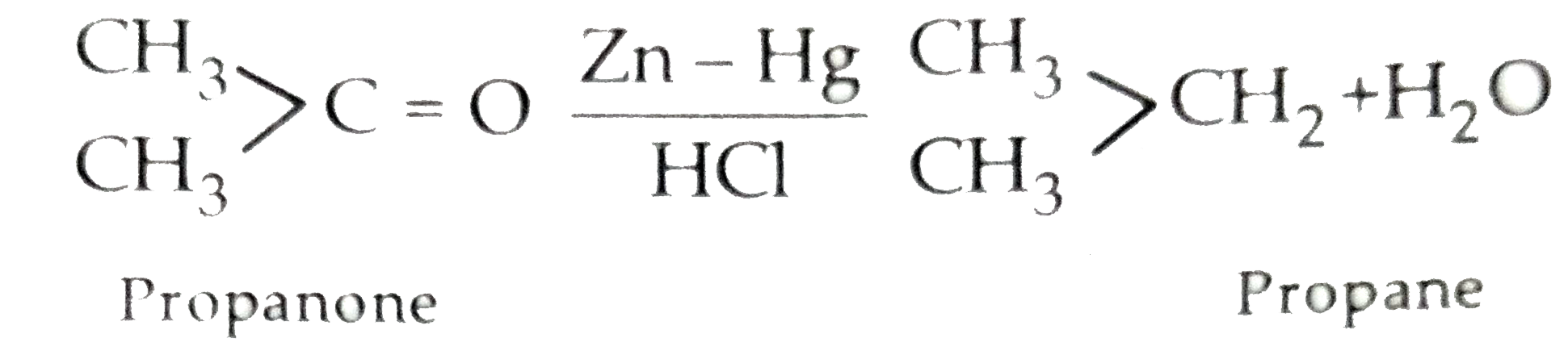Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- (a) Illustrate the following name reactions by giving example : (i) ...
Text Solution
|
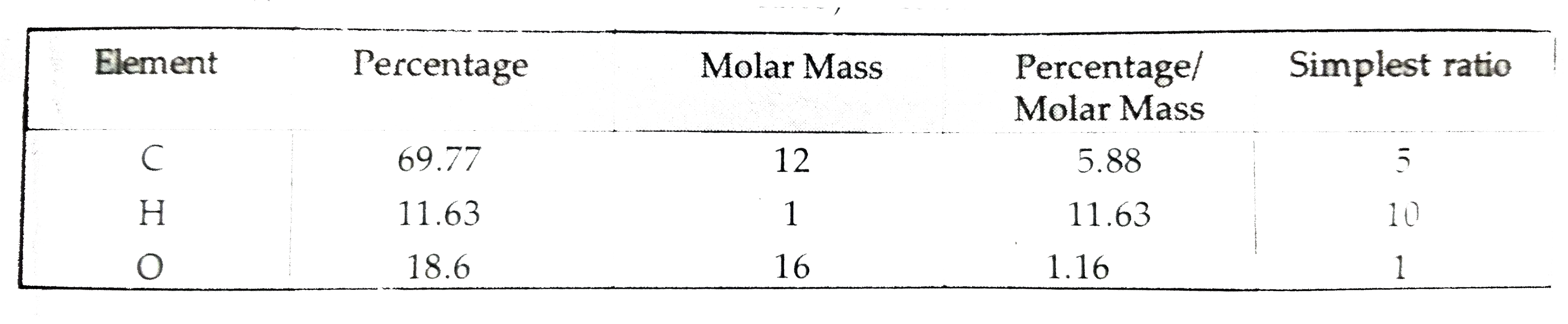
- An organic compound contains 69.77% carbon, 11.63% hydrogen, and rest ...
Text Solution
|
- An unknown compound of carbon , hydrogen and oxygen contains 69.77 % C...
Text Solution
|
- An organic compound A contains 69.77% carbon, 11.63% hydrogen and the ...
Text Solution
|
- (a) Illustrate the following name reactions by giving example : (i) Ca...
Text Solution
|
- एक कार्बनिक यौगिक में 69.77% कार्बन, 11.63% हाइड्रोजन तथा शेष ऑक्सीजन ...
Text Solution
|
- एक कार्बनिक यौगिक में 69.77% कार्बन 11.63% हाइड्रोजन तथा शेष ऑक्सीजन ह...
Text Solution
|
- An organic compound contains 69.77% carbon, 11.63% hydrogen and rest o...
Text Solution
|
- एक कार्बनिक यौगिक में 69.77% कार्बन ,11.63% हाइड्रोजन तथा शेष ऑ...
Text Solution
|