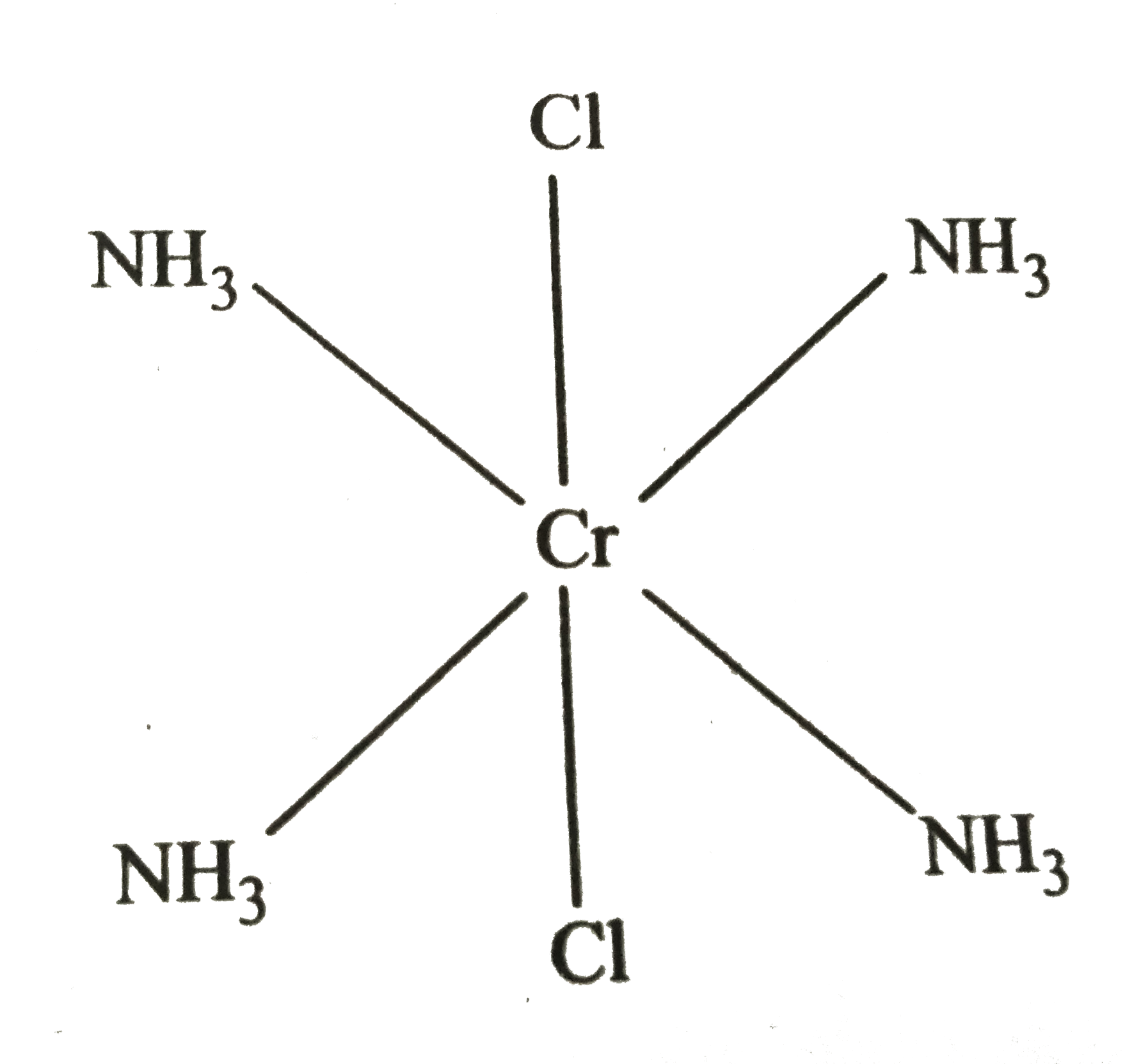
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-Outside Delhi : SET-III
- What are n-type semiconductors ?
Text Solution
|
- What is the basicity of H(3)PO(2) acid and why ?
Text Solution
|
- Write the IUPAC name of the following :
Text Solution
|
- How do you explain the presence of all the six carbon atoms in glucose...
Text Solution
|
- What is the cause of a feeling of depression in human beings ? Name a ...
Text Solution
|
- The molar conductivity of a 1.5 M solution of an electrolyte is found ...
Text Solution
|
- Explain the role of each of the following : (i) NaCN in the extracti...
Text Solution
|
- Diffferentiate between disinfectants and antiseptics . Give one exampl...
Text Solution
|
- The electrical resistance of a column of 0.05 " mol " L^(-1) NaOH solu...
Text Solution
|
- Write three distinct features of chemisorptions which are not found in...
Text Solution
|
- How would you account for the following ? (i) With the same d-orbit...
Text Solution
|
- Name of following coordinatin entities and describe their structures :...
Text Solution
|
- Define the following as related to proteins : (i) Peptide linkage ...
Text Solution
|