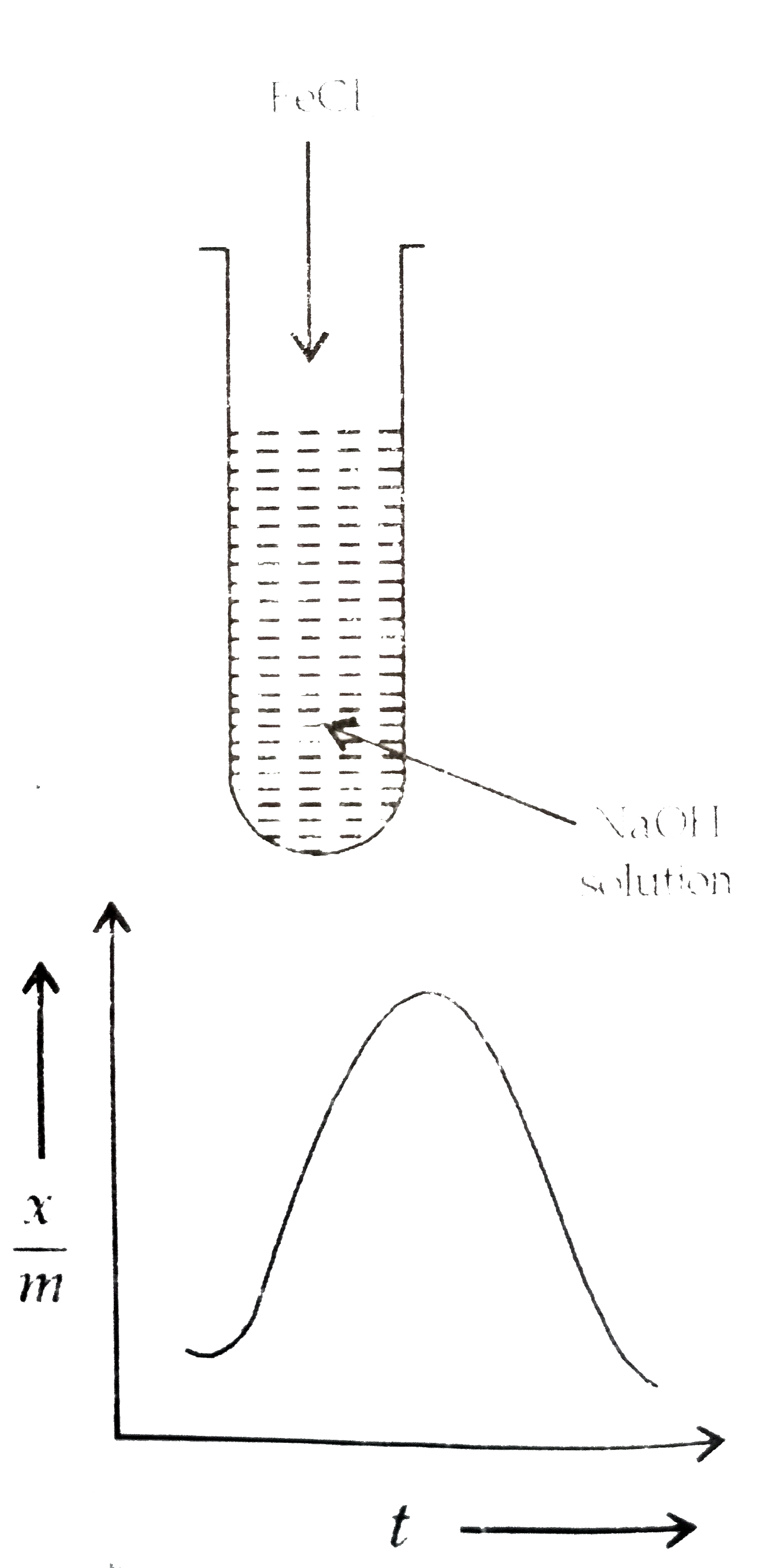
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-SECTION-C
- The decomposition of NH, on platinum surface is zero order reaction. I...
Text Solution
|
- (i) What is the role of activated charcoal in gas mask ? (ii) A coll...
Text Solution
|
- An element crystallizes in fec lattice with a cell edge of 300 pm. The...
Text Solution
|
- A 4% solution (w/w) of sucrose (M 342 g mol^(-1)) in water has a freez...
Text Solution
|
- (a) Name the method of refining which is (i) used to obtain semicond...
Text Solution
|
- Give reasons for the following: (i) Transition elements and their co...
Text Solution
|
- Write the structures of monomers used for getting the following polyme...
Text Solution
|
- (i) [-CH(2)-overset(CH(3))overset(|)(C)H-](n) a homopolymer or copoly...
Text Solution
|
- (i) What type of drug is used in sleeping pills? (ii) What type of d...
Text Solution
|
- Define the following terms with a suitable example in each: (i) Broa...
Text Solution
|
- (i) Out of (CH(3))(3), C-Br and (CH(3))(3) C-I, which one is more reac...
Text Solution
|
- An aromatic compound A on heating with Br, and KOH forms a compound B ...
Text Solution
|
- Complete the following reactions: (i) (ii) (C(6)H(5)CH(2))(2)Cd+2...
Text Solution
|
- Write chemical equations for the following reactions: (i) Propanone ...
Text Solution
|
- Differentiate between the following: (i) Amylose and Amylopectin. ...
Text Solution
|
- Write chemical reaction to show that open structure of D-glucose conta...
Text Solution
|
- Chromium metal can be plated out from an acidic solution containing Cr...
Text Solution
|
- Give reasons for the following : (a) Leather gets hardened after tan...
Text Solution
|
- What is the role of : ( i) Depressants in froth floatation ? (b) Ca...
Text Solution
|
- Write chemical reactions taking place in the extraction of Aluminium f...
Text Solution
|