Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-SECTION-D
- E(cell)^(@) for the given redox reaction is 2.71 V Mg((s))+Cu((0.01 ...
Text Solution
|
- (a) A steady current of 2 amperes was passed through two electrolytic ...
Text Solution
|
- (a) How do you convert the following: (i) Phenol to Anisole (ii) Eth...
Text Solution
|
- (a) Account for the following : (i) o-nitrophenol is more steam vola...
Text Solution
|
- (a) Give reasons for the following: (i) Sulphurin vapour state shows...
Text Solution
|
- (a) (i) Write the disproportionation reaction of H(3)PO(3) (ii) Draw...
Text Solution
|
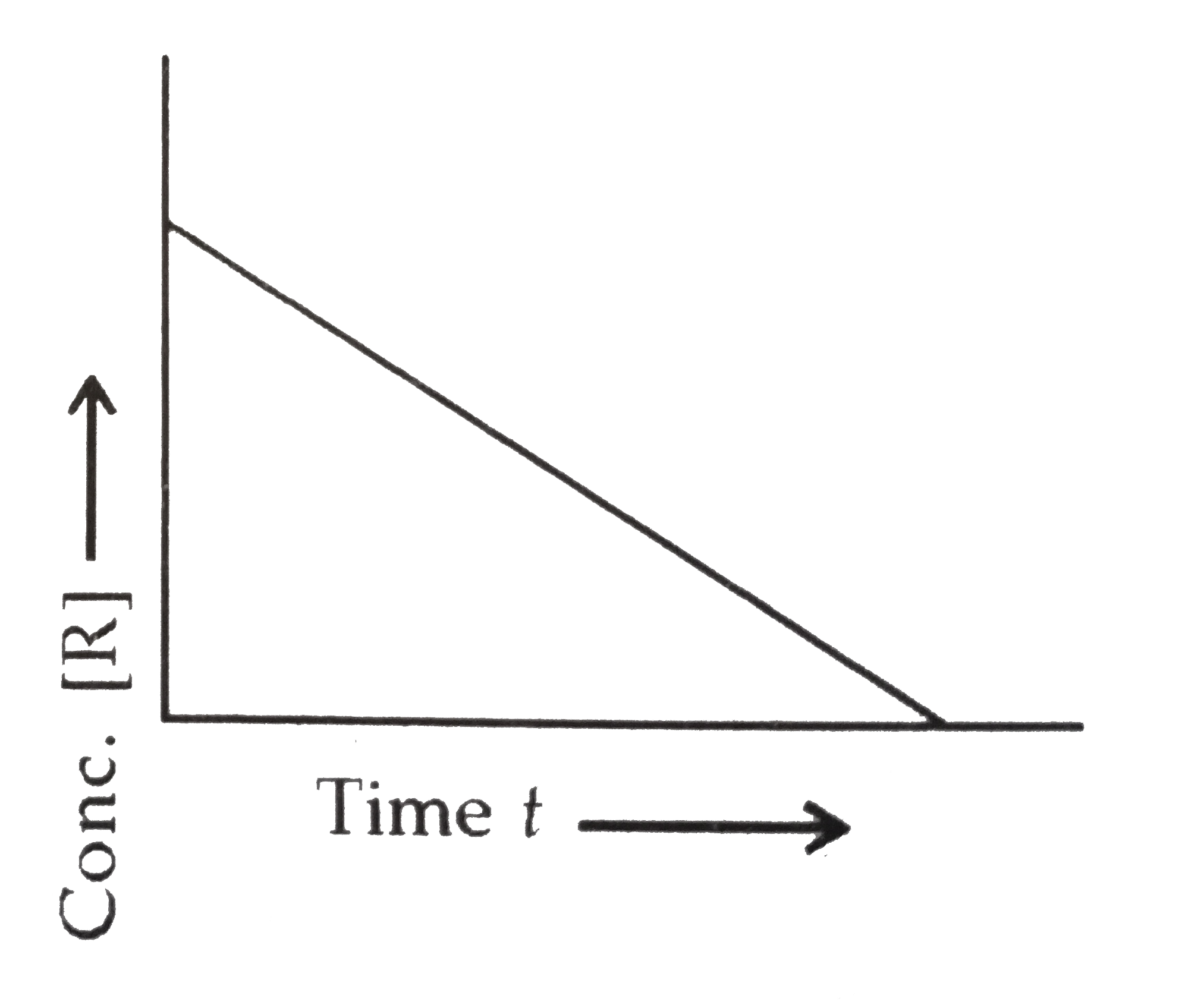
- Consider the reaction R rarr P for which the change in concentration o...
Text Solution
|
- Draw the plot of In vs 1/T for a chemical reaction .What does the inte...
Text Solution
|
- Draw the structure of the following (i) HClO3 (ii) H2S2O8 ( b) ...
Text Solution
|
- Complete the following reactions: (i) PbS(s)+O3rarr (ii) XeF6+NaF ra...
Text Solution
|
- Carry out the following conversions : (i) pnitrotoluene to 2-bromob...
Text Solution
|
- Carry out the following conversions : (i) Benzoic acid to aniline ....
Text Solution
|