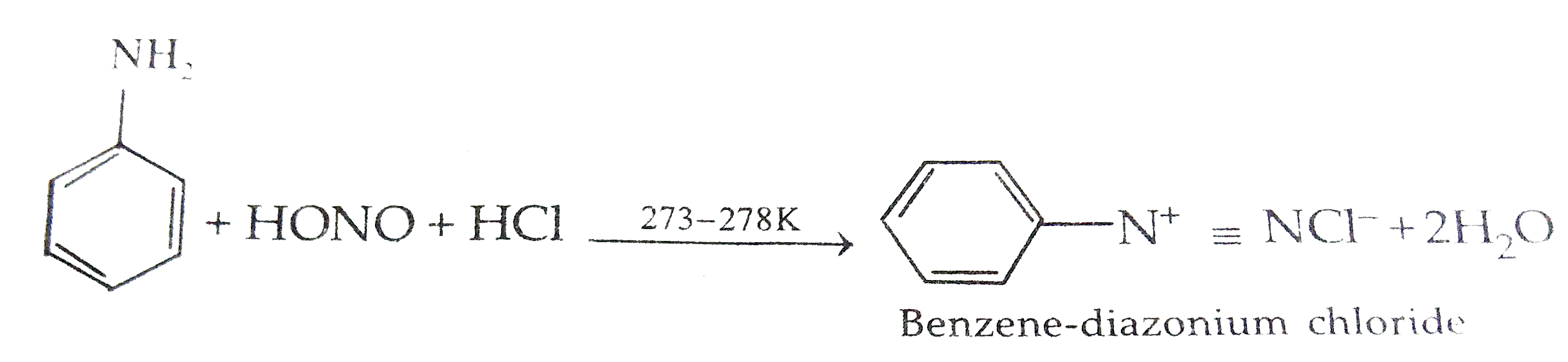
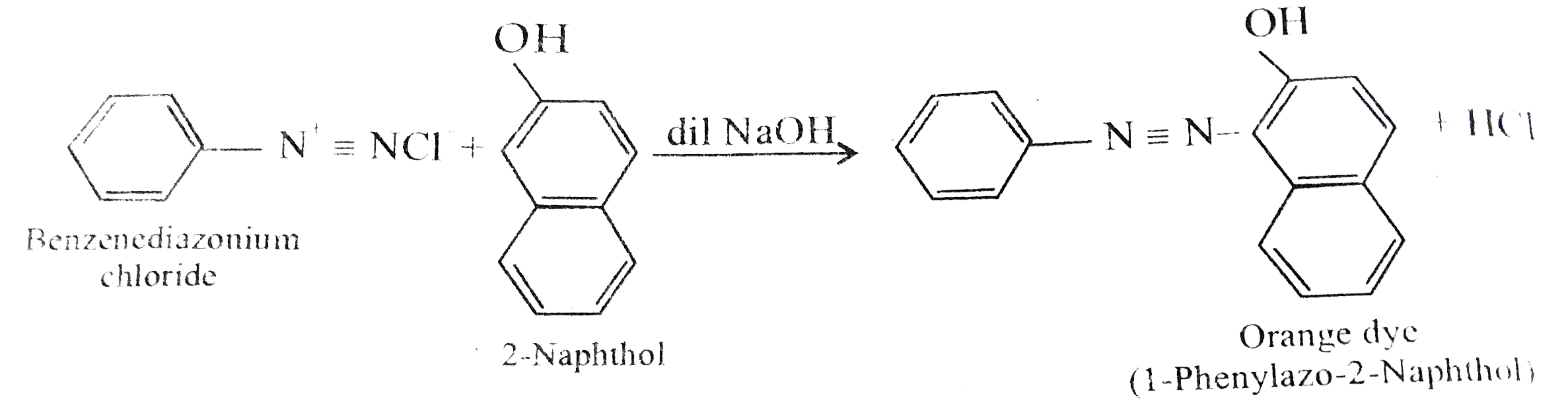
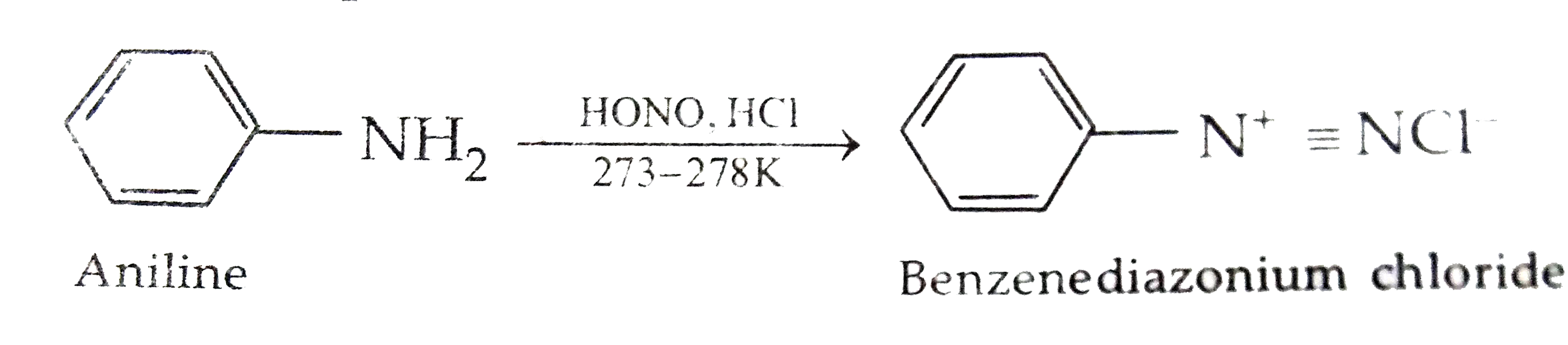
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-CHEMISTRY (Theory) [SET - I]
- Complete the following reactions (i) C(2)H(5)OC(2)H(5)+HCl to (ii...
Text Solution
|
- Draw the structural formulae of the following compounds : (i) H(4)P(...
Text Solution
|
- Give the chemical tests to distinguish between the following pairs of ...
Text Solution
|
- Identify A and B in each of the following processes : (i) CH(3)CH(2...
Text Solution
|
- Draw the structures of the monomers of the following polymers: (i) Po...
Text Solution
|
- The density of copper metal is 8.95" g "cm^(-3). If the redius of copp...
Text Solution
|
- What mass of NaCl ("molar mass" =58.5g mol^(-1)) be dissolved in 65g o...
Text Solution
|
- Describe the role of the following : (i) NaCl in the extraction of ...
Text Solution
|
- Describe the principle involved in each of the following processes of ...
Text Solution
|
- Why is europium (II) more stable than cerium (II) ?
Text Solution
|
- Explain the mechanism of S(N^(1)) and S(N^(2)) reactions with examples...
Text Solution
|
- How would you convert the following : (i)Phenol to benzoquinone (i...
Text Solution
|
- Explain the following : (a) The electron gain enthalpy with negative...
Text Solution
|
- Anino acids may be acidic, alkaline or neutral, How does this happen? ...
Text Solution
|
- Explain the following terms with one example in each case : (i) Foo...
Text Solution
|
- (a) Explain the following terms : (i) Rate of a reaction (ii) Act...
Text Solution
|
- (a) Explain the following terms : (i) Order of a reaction (ii) Mol...
Text Solution
|
- (a) Complete the following chemical equations: (i) Cr(2) O(7)^(2-)...
Text Solution
|
- (a) Complete the following chemical equations : (i) MnO(4)^(-) (aq) ...
Text Solution
|
- (a) Illustrate the following name reactions giving a chemical equation...
Text Solution
|