Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-[OUTSIDE DELHI : SET -II]
- Which point defect in crystals of a solid decreases the density of the...
Text Solution
|
- What is the primary structural feature necessary for a molecule to m...
Text Solution
|
- Iron has body centred cubic cell with a cell edge of 286.5 pm. The den...
Text Solution
|
- For a decomposition reaction the values of rate constant k at two diff...
Text Solution
|
- Complete the following reaction equations : (i) C(6)H(5)N(2)Cl+CH...
Text Solution
|
- Give chemical tests to distinguish between compounds in the following...
Text Solution
|
- (a) Arrange the following in an increasing order of their indicated p...
Text Solution
|
- Write down the electronic configuration of : (a) Cr^(3+) (b) ...
Text Solution
|
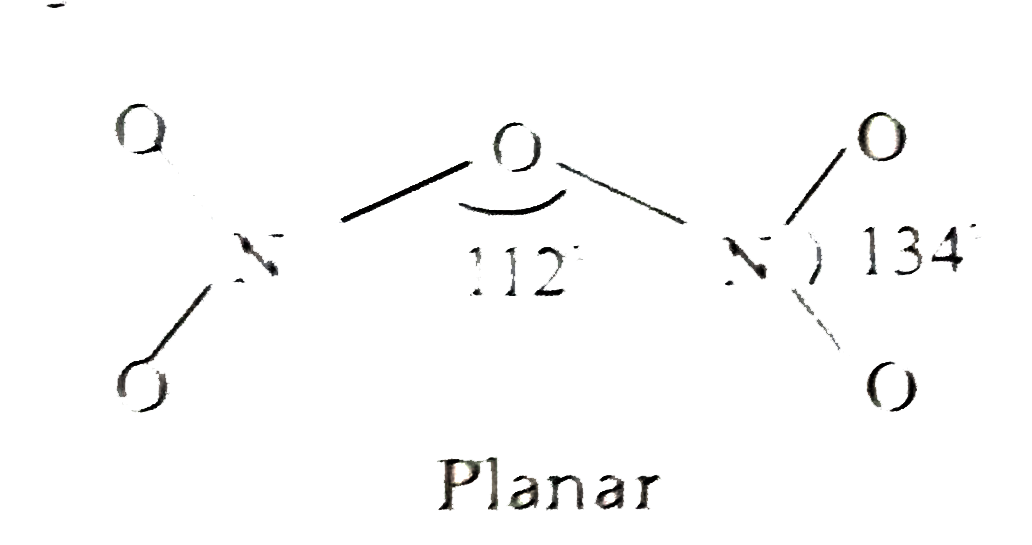
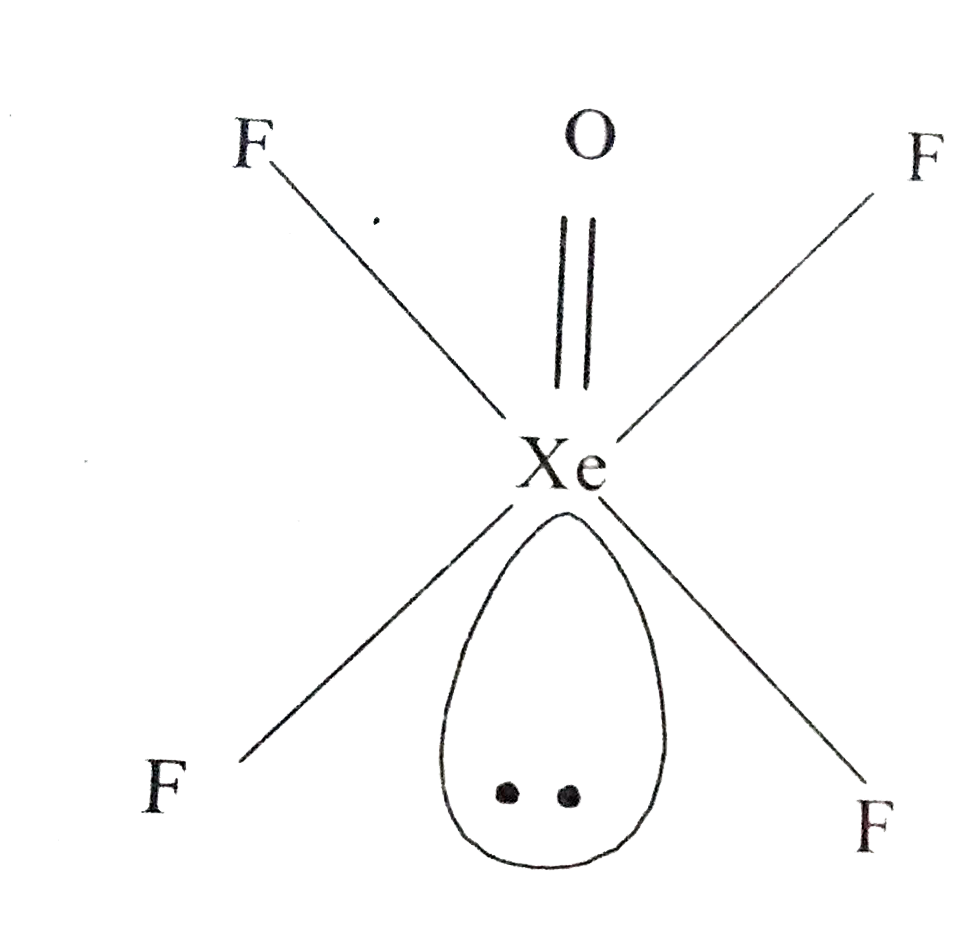
- Draw the structures of the following : (i) N(2)O(5) (ii) XeOF(4...
Text Solution
|