Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-[OUTSIDE DELHI : SET -III]
- How many atoms constitute one unit cell of a face-centred cubic crysta...
Text Solution
|
- Describe the role of the following : (i) NaCN in the extraction o...
Text Solution
|
- Define " Order of a reaction" and " Activation energy of a reaction "...
Text Solution
|
- Differentiate between the molecular structures and behaviour of thermo...
Text Solution
|
- A voltaic cell is set up at 25^(@)Cwith the following half cells : A...
Text Solution
|
- Explain the following : (i) Low spin octahedral complexes of nicke...
Text Solution
|
- Compare the following complexes with respect to structural shapes of u...
Text Solution
|
- What are the following substances " Given one example of each of them...
Text Solution
|
- Draw the structures of the following : (i) XeF(4) (ii) H(2)S(2...
Text Solution
|
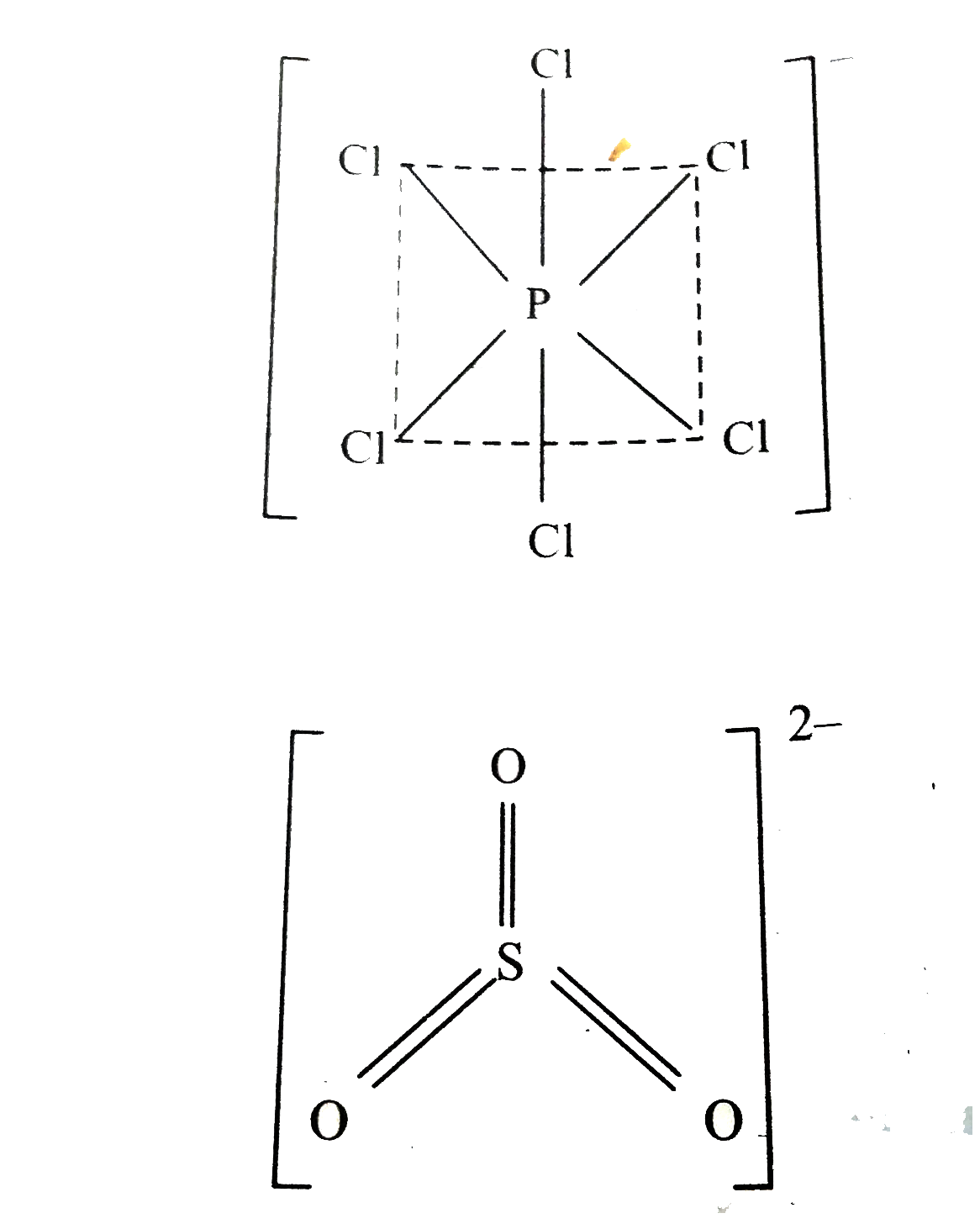
- (a) Draw the structures of the following : (i) PCl(5)(s) (ii) ...
Text Solution
|