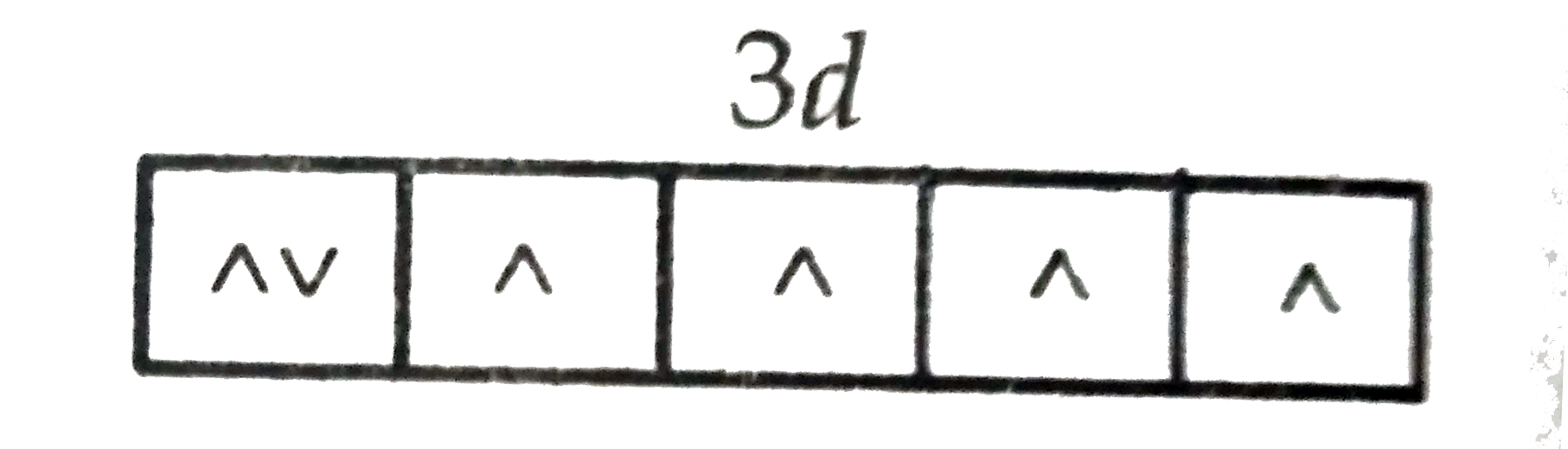Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-[SET-1]
- An element exists in bcc lattice with a cell edge of 288 pm. Calculate...
Text Solution
|
- Calculate Delta(r)G^(@) and log K(c) for the following reaction at 29...
Text Solution
|
- For a first order reaction, show that the time required for 99% comple...
Text Solution
|
- Explain the following phenomenon given reason : (i) Tnndall effect. ...
Text Solution
|
- (a) Write the principle involved in the following : {:((i)"Zone refi...
Text Solution
|
- A mixed oxide of iron and chromium is fused with sodium carbonate in f...
Text Solution
|
- (a) Give reasons for the following : (i) Compounds of transition ele...
Text Solution
|
- For the complex ion [F(en)(2)CI(2)]^(+) write the hybridization type a...
Text Solution
|
- (a) Account for the following : (i) Electrophilic substitution react...
Text Solution
|
- (a) Why phenol is more acidic than than ethanol ? (b) Write the mech...
Text Solution
|
- Identify A, B and C in the following reactions : (i) CH(3)CH(2)Clove...
Text Solution
|
- (a) Why water soluble vitamins must be supplied regularly in the dite ...
Text Solution
|
- (i) Name a substance which can be used as an antiseptic as well as dis...
Text Solution
|
- Once there was heavy downpour for about 3 hours in the early morning. ...
Text Solution
|
- (a) Explain why on addition of 1 mole glucose to 1 litre water the bol...
Text Solution
|
- (a) Define the following terms : {:((i)"Ideal solution",(ii)"Osmitic...
Text Solution
|
- (a) When concentrated sulphuric acid was added to an unknown salt pres...
Text Solution
|
- Account for the following (i) Reducing character decreases from SO(2...
Text Solution
|
- (a) Account for the following : (i) Propanal is more reactive than p...
Text Solution
|
- Write structure of A, B C and D in the following eactin sequence : {...
Text Solution
|