Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
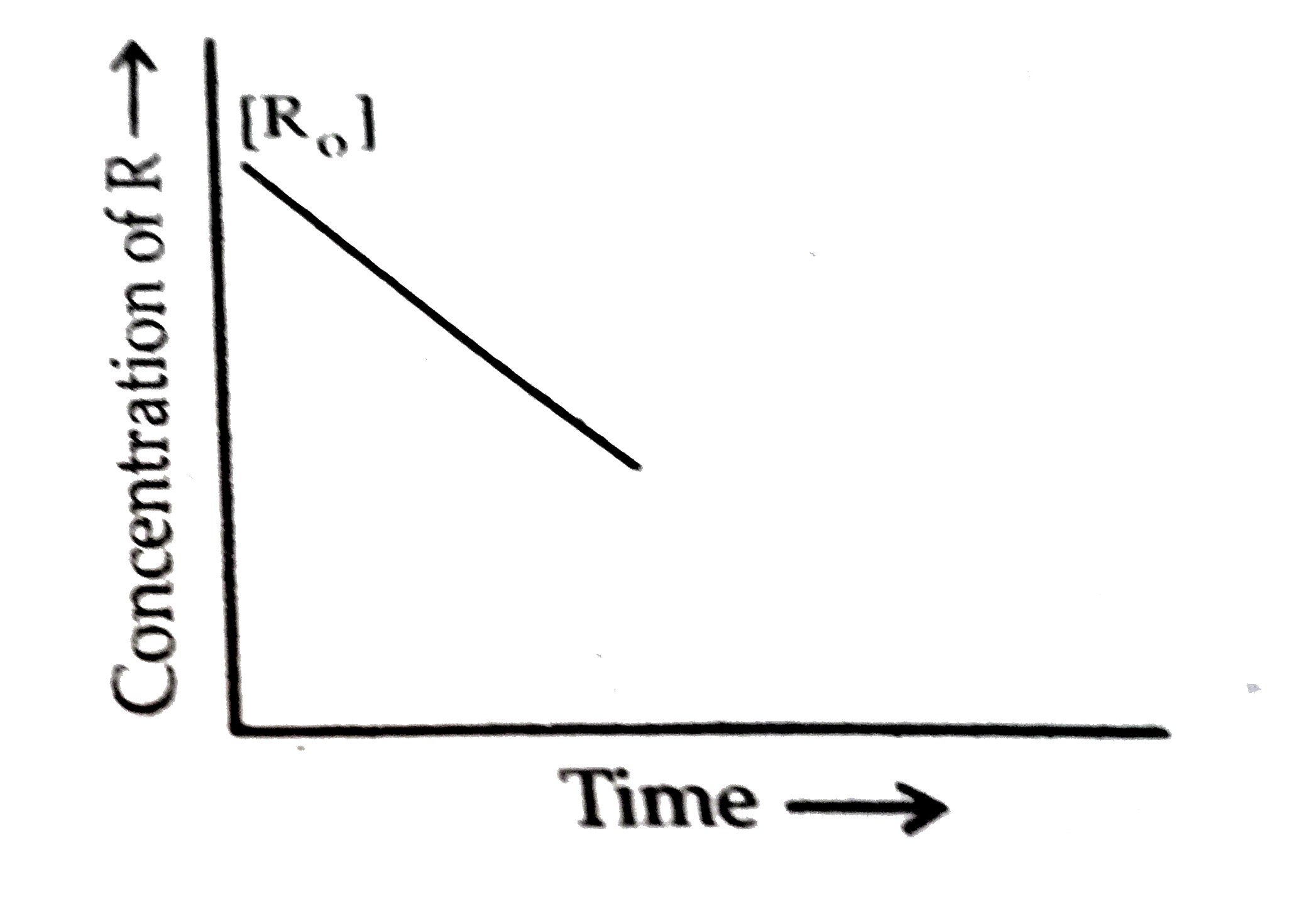
- For a chemical reactin R rarr P, variation of concentration of R vs ti...
Text Solution
|
- For a chemical reaction, variation in concentration [A] vs time (s) pl...
Text Solution
|
- For a chemical reaction, variation in concentration , in [R] vs time (...
Text Solution
|
- For chemical reaction R rarr P the variation in the concentration (R )...
Text Solution
|
- For a certain chemical reaction variation in concentration [A] vs. tim...
Text Solution
|
- For a chemical reactin R rarr P, variation of concentration of R vs ti...
Text Solution
|
- For a chemical reaction R to P, the variation in the concentration (R)...
Text Solution
|
- For a chemical reaction R to P, the variation in the concentration (R)...
Text Solution
|
- For a chemical reaction R to P, the variation in the concentration (R)...
Text Solution
|