Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
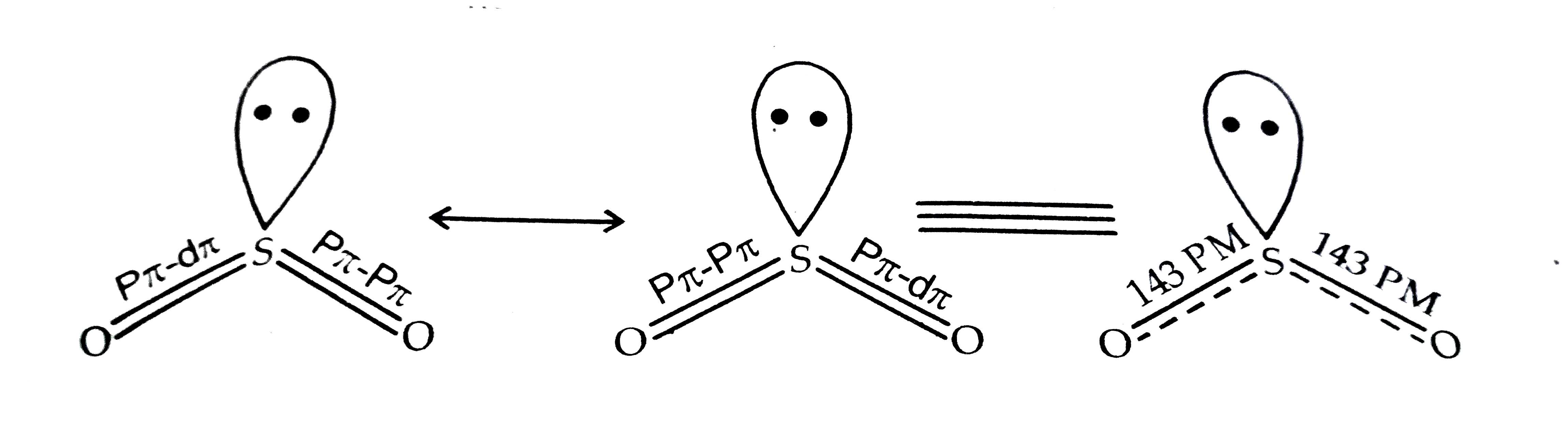
- Account for the following : Two S - O bond lengths in SO(2) are equ...
Text Solution
|
- Comment on the nature of two S–O bonds formed in SO(2) molecule. Are t...
Text Solution
|
- Account for the following : Two S - O bond lengths in SO(2) are equal....
Text Solution
|
- फ्लोरीन के सभी यौगिकों में इसकी ऑक्सीकरण अवस्था केवल - 1 होती है, क्यो...
Text Solution
|
- Comment on the nature of two S - O bonds formed is SO(2) molecule. Are...
Text Solution
|
- Comment on the nature of two S–O bonds formed in SO(2) molecule. Are t...
Text Solution
|
- The two O-O bond lengths in ozone molecule are equal. Assign reason.
Text Solution
|
- फ्लुओरीन केवल -1 ऑक्सीकरण अवस्त्था ही दर्शाता है , क्यों ?
Text Solution
|
- Comment on the nature of two S-O bonds formed in SO(2) molecule. Are t...
Text Solution
|