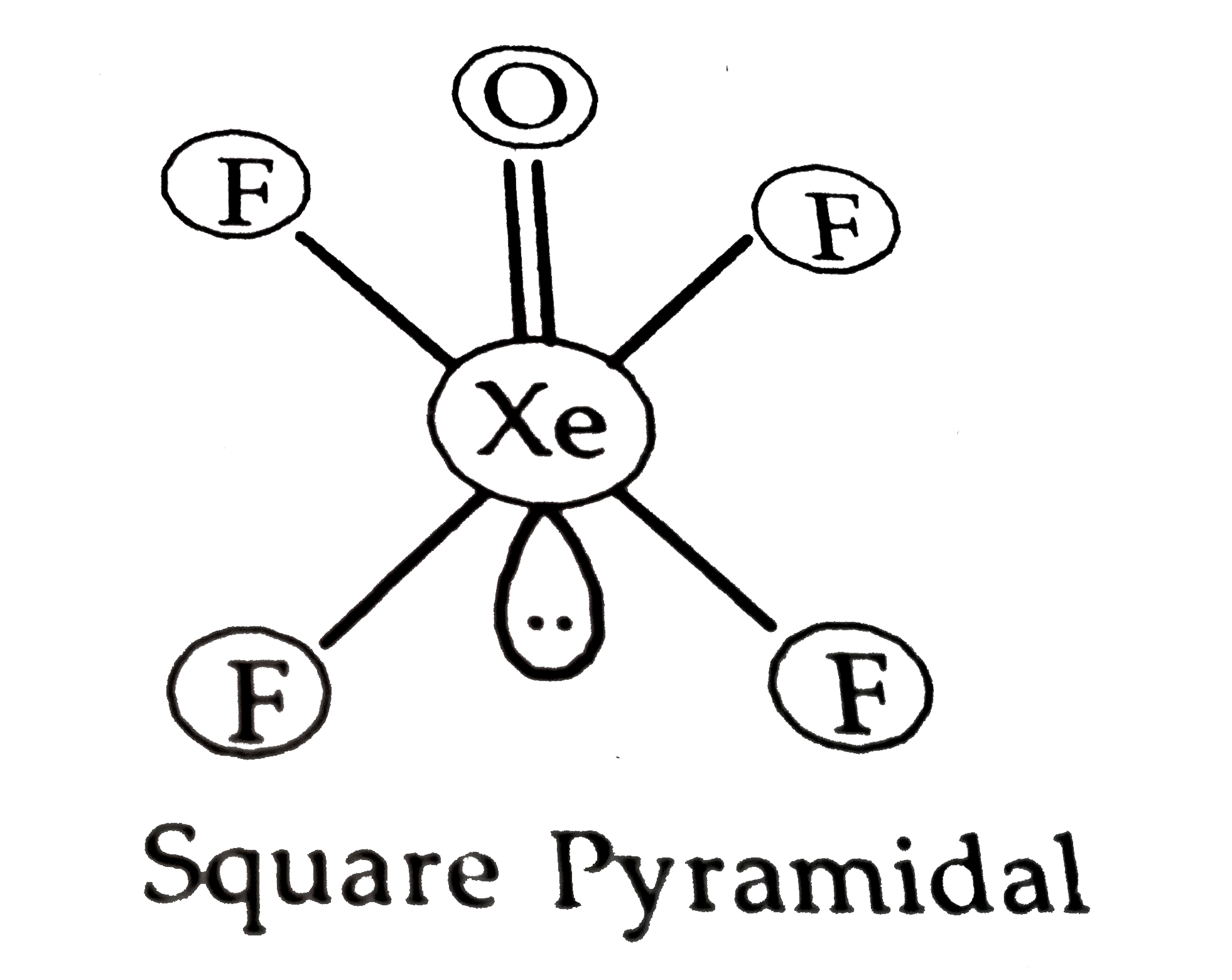
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- (a) Arrange the hydrides of group 16 in increasing order of the acidic...
Text Solution
|
- Arrange the hydrides of group 16 elements in order of increasing boili...
Text Solution
|
- Arrange the hydrides of group 16 elements in increasing order of reduc...
Text Solution
|
- Arrange the hydrides of group 16 elements in order of increasing acid ...
Text Solution
|
- (a) Arrange the hydrides of group 16 in increasing order of the acidic...
Text Solution
|
- उपयुक्त कारण देते हुए समूह 16 के तत्वों के हाइड्राइडों को उनके अम्लीय...
Text Solution
|
- Arrange the following hydrides of group 16 elements in order of increa...
Text Solution
|
- Arrange the hydrides of the elements of group -16 in the order of...
Text Solution
|
- Arrage the group - 16 hydrides in the order of increasing acidic stren...
Text Solution
|