Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
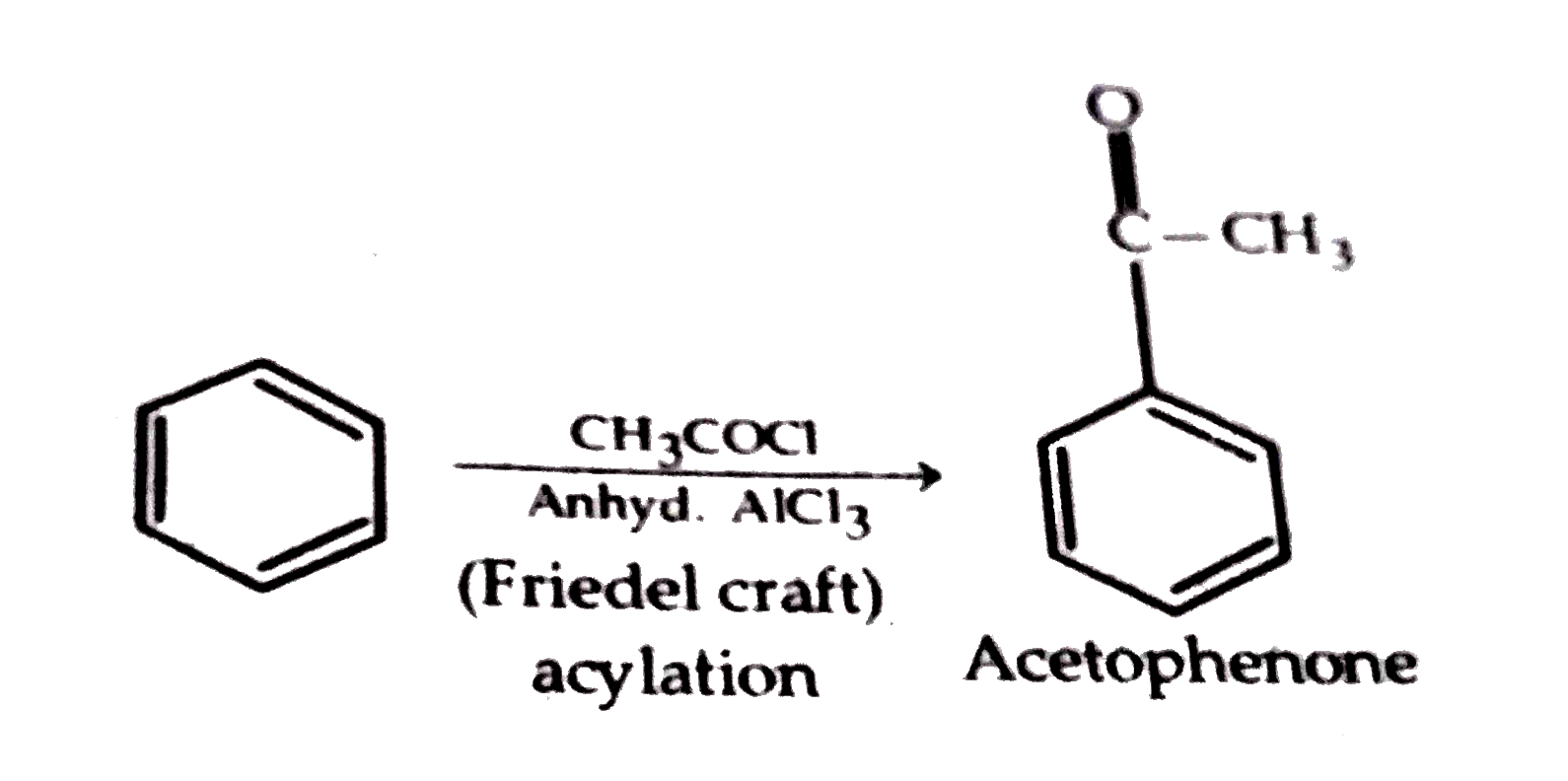
- (a) How will you convert (i) Benzene to acetophenone (ii) Propan...
Text Solution
|
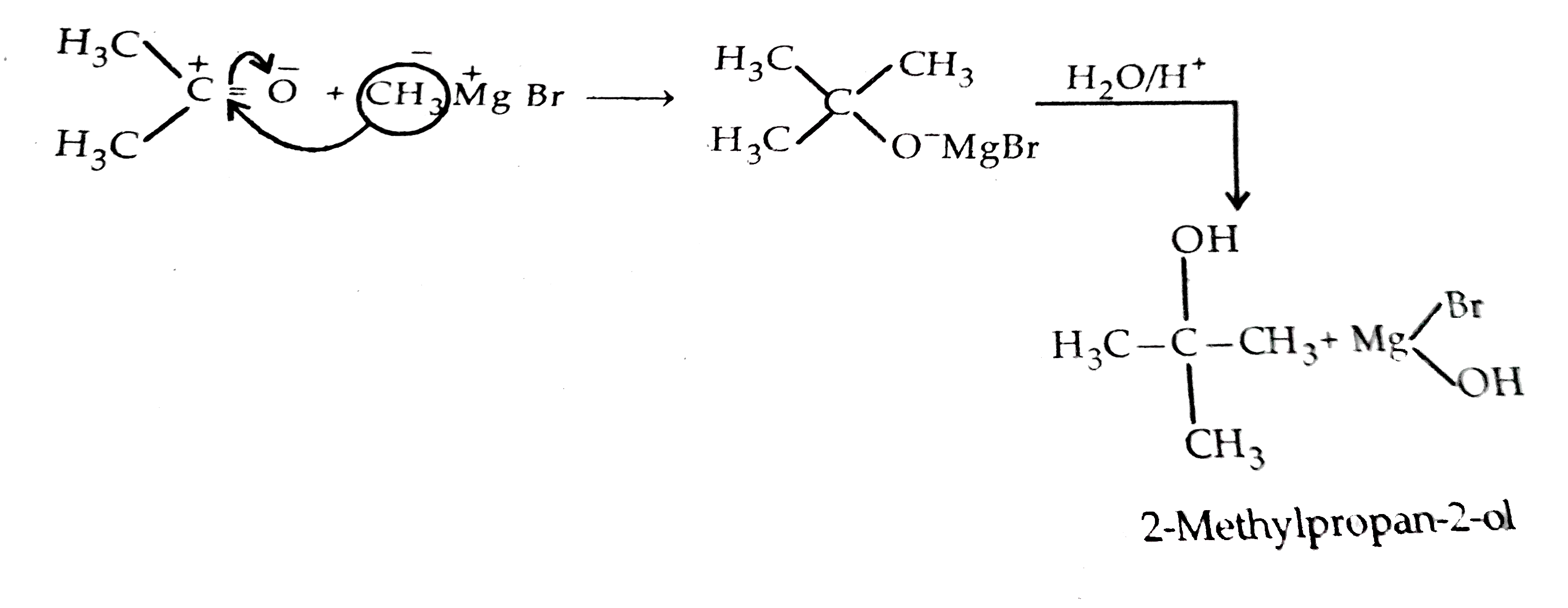
- How will you convert propanone to propan-2-ol?
Text Solution
|
- Explain giving reasons each of the following : (i) Chloroacetic acid...
Text Solution
|
- How would you convert the following : (i)Phenol to benzoquinone (ii) P...
Text Solution
|
- (a) Account for the following : (i) Propanal is more reactive than p...
Text Solution
|
- (a) How will you convert (i) Benzene to acetophenone (ii) Propan...
Text Solution
|
- (a) How many you account for the following : (i) Aldehydes are more re...
Text Solution
|
- How will you convert propanone to propan-2-ol?
Text Solution
|
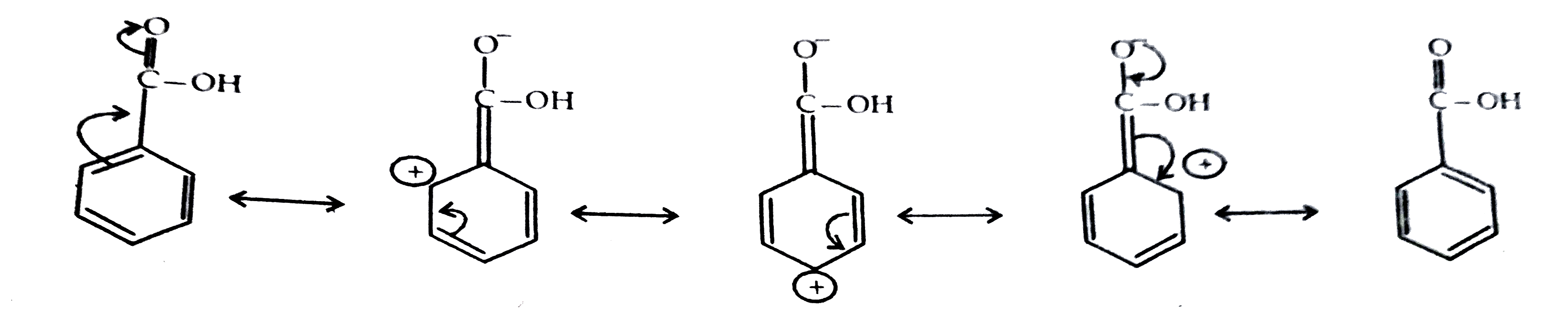
- Carboxylic acids have higher boiling point than aldehyde, keton and ev...
Text Solution
|