Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
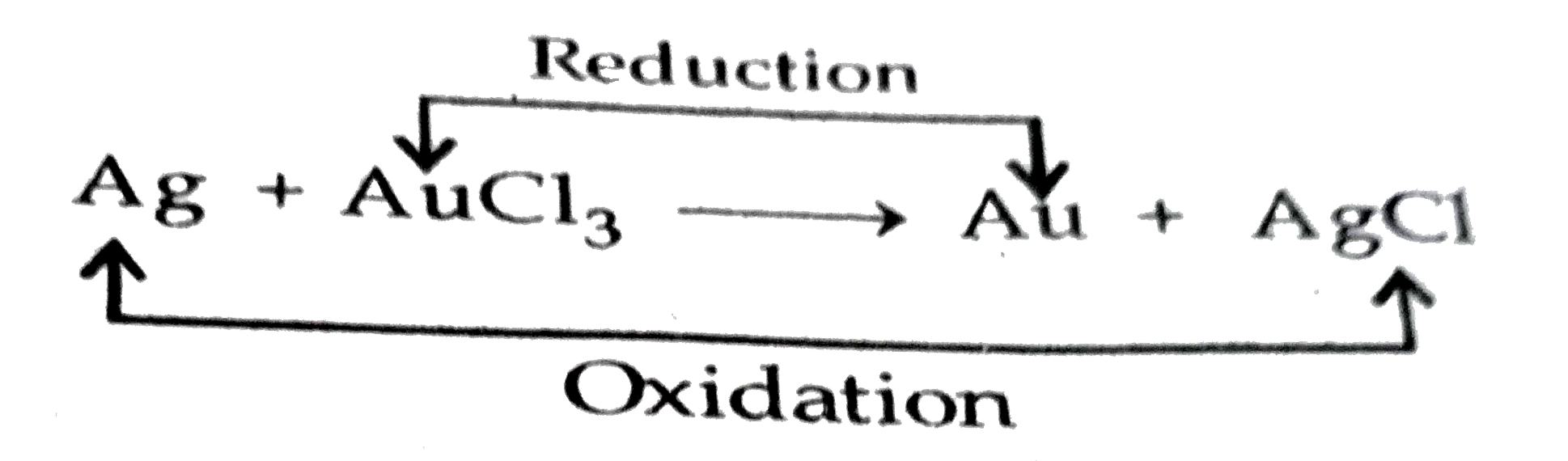
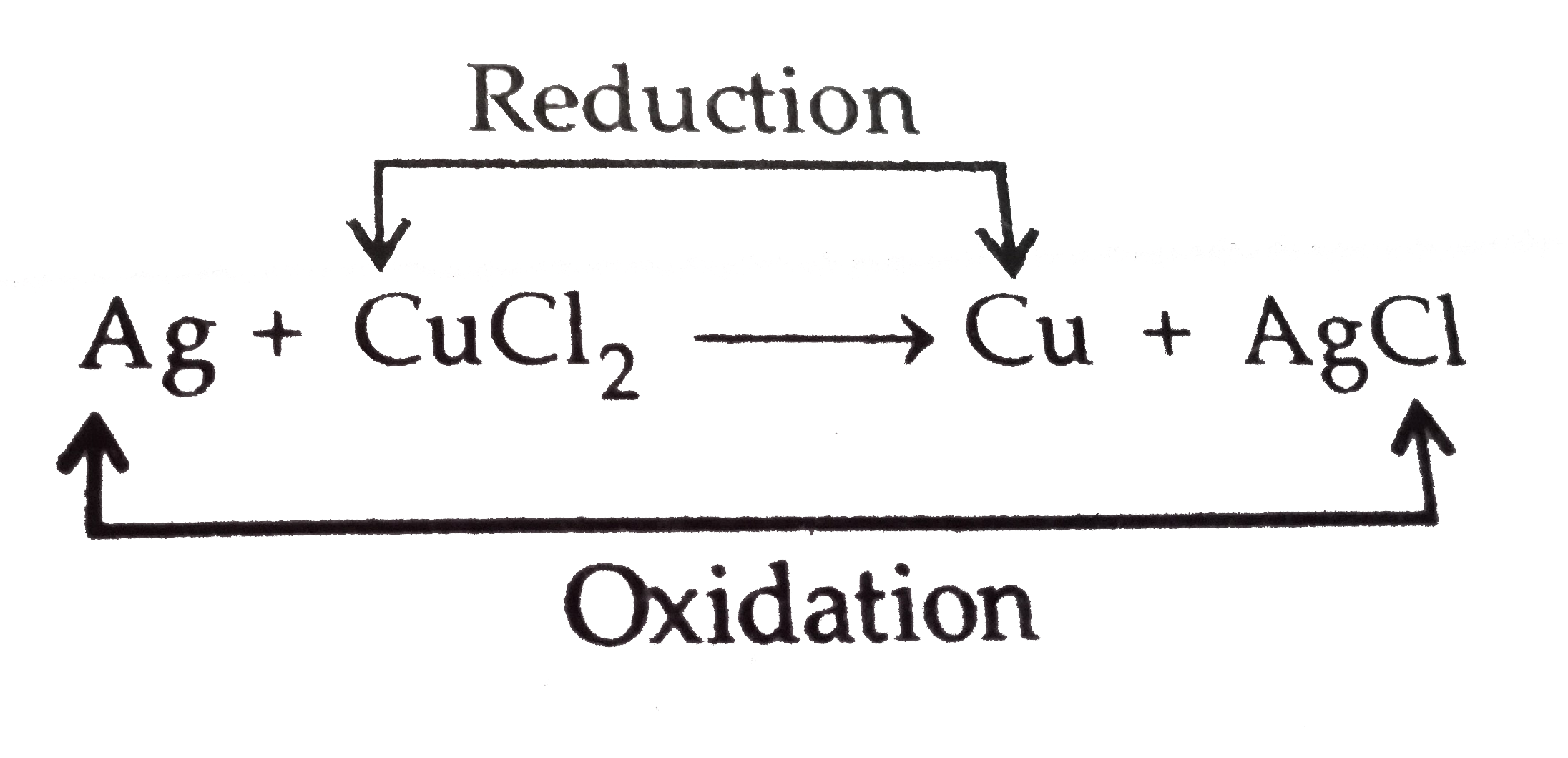
- (a) When a bright silver object is placed in the solution of gold chlo...
Text Solution
|
- Calculate the emf of the following cell: Cu(s)|Cu^(2+) (aq)||Ag^(+) (a...
Text Solution
|
- A copper-silver cell is set up. The copper ion concentration in it is ...
Text Solution
|
- (a) When a bright silver object is placed in the solution of gold chlo...
Text Solution
|
- Is it safe to stir 1M AgNO(3) solution with copper spoon? Given:E^(@)A...
Text Solution
|
- Can we use a copper vessel to store 1 M AgNO3 solution? Given that E(...
Text Solution
|
- Is it possible to store : (i) copper suplhate solution in a zonc vesse...
Text Solution
|
- Can we use a copper vessel to store 1 M AgNO(3) solution ? Given that ...
Text Solution
|
- Is it possible to store : (i) copper suplhate solution in a zonc vesse...
Text Solution
|