Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
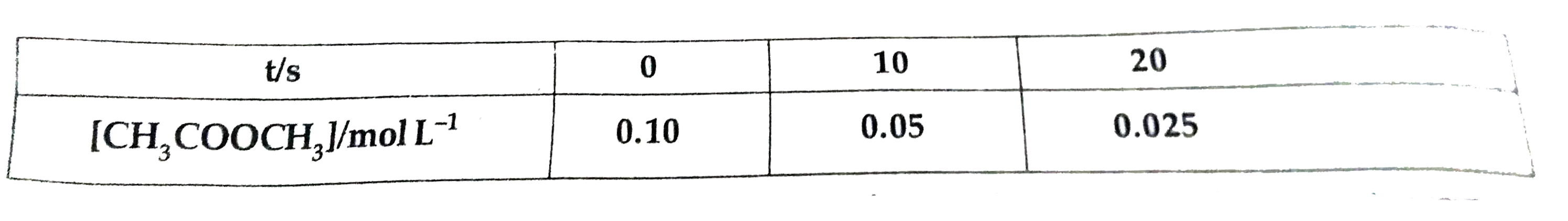
- For the hydrolysis of methyl acetate in aqueous solution, the followin...
Text Solution
|
- For the hydrolysis of methyl acetate in aqueous solution, the followin...
Text Solution
|
- In a pseudo first order hydrolysis of ester in water, the following re...
Text Solution
|
- For the hydrolysis of methyl acetate in aqueous solution, the followin...
Text Solution
|
- For the hydrolysis of methyl acetate in aqueous solution, the followin...
Text Solution
|
- (a) जलीय विलयन में मेथिल ऐसीटेट के जल-अपघटन से निम्नलिखित परिणाम प्राप...
Text Solution
|
- समयांतराल 10 से 20 सेकेण्ड के बीच अभिक्रिया की औसत दर परिकलित कीजिये। ...
Text Solution
|
- In a pseudo first order hydrolysis of ester in water the following res...
Text Solution
|
- In a pseudo first order reaction of hydrolysis of an ester in H2O, the...
Text Solution
|