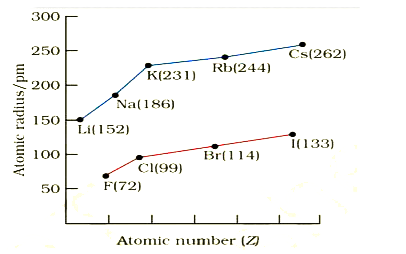
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
CBSE MODEL PAPER-SAMPLE QUESTION PAPER 2020-21 (SCIENCE)-SECTION D
- Metallic Character The ability of an atom to donate electrons and fo...
Text Solution
|
- Match the following pH values 1, 7, 10, 13 to the solutions given belo...
Text Solution
|
- (i) Four samples A, B, C and D change the colour of pH paper or soluti...
Text Solution
|
- Trace the changes that take place in a flower from gamete formation to...
Text Solution
|
- In the given circuit, A, B, C and D are four lamps connected with a ba...
Text Solution
|
- PQ is a current carrying conductor in the plane of the paper as shown ...
Text Solution
|