Text Solution
Verified by Experts
CBSE MODEL PAPER-SAMPLE QUESTION PAPER 2020-21 (SCIENCE)-SECTION C
- In humans, there is a 50% probability of the birth of a boy and 50% pr...
Text Solution
|
- Plastic cups were used to serve tea in trains in early days- these cou...
Text Solution
|
- Explain where and how urine is produced?
Text Solution
|
- a. Which of the following reactions is/ are an endothermic reaction(s)...
Text Solution
|
- The following table shows the position of five elements A, B, C, D and...
Text Solution
|
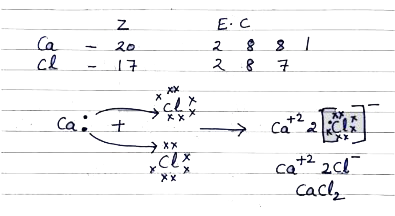
- a. Explain the formation of Calcium Chloride with the help of electron...
Text Solution
|
- Refractive index of water with respect to air is 1.33 and that of diam...
Text Solution
|