Similar Questions
Explore conceptually related problems
Recommended Questions
- The standard reduction potential at 25^(@)C for the reaction, volt...
Text Solution
|
- The standard potential of a cell using the reaction +3HgO(s)+2(overse...
Text Solution
|
- The standard reduction potential at 25^(@)C for the reaction, volt. Th...
Text Solution
|
- एक सेल अभिक्रिया जिसमे दो इलेक्ट्रॉनों का परिवर्तन होता है में सेल का ...
Text Solution
|
- 25^(@)C पर एक सैल , जिसकी सैल अभिक्रिया के दौरान 2 एलेक्ट्रॉनों का पर...
Text Solution
|
- At 25^(@)C , the standard free energy change for a reaction is 5.4 kJ....
Text Solution
|
- The standard reduction potential at 25^(@)C for the reduction of water...
Text Solution
|
- The standard reduction potential at 25^(@)C of the reaction 2H(2)O+2...
Text Solution
|
- गैल्वनिक सेल की सेल अभिक्रिया, (n=2) के मानक विद्युत वाहक बल का मान 25...
Text Solution
|
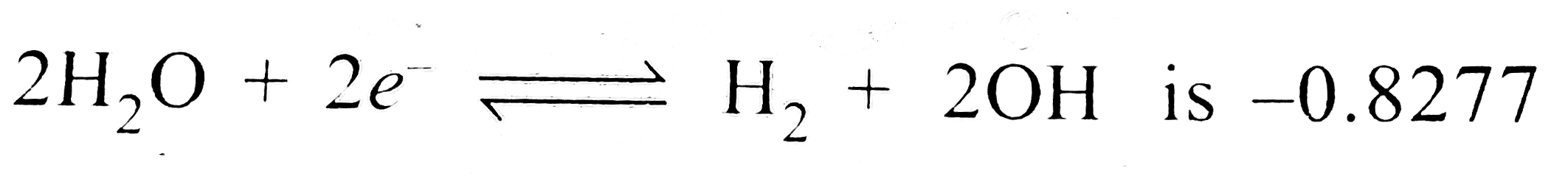 volt. The equilibrium constant for the reaction `:`
volt. The equilibrium constant for the reaction `:`  is
is