Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE|Exercise Exercise-3 Part-I: JEE(Advance ) /IIT-JEE Problem (Comprehenion )|1 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Exercise-3 Part-II: JEE(Main ) /AIEEE Problem|36 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Exercise-2 Part-IV: Comprehension -5|1 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(d & f- Block Elments)|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-COORDINATION COMPOUNDS-Exercise-3 Part-I: JEE(Advance ) /IIT-JEE Problem
- The species having tetrahedral shape is :
Text Solution
|
- The spin magnetic moment of cobalt in the compound , Hg[Co(SCN)(4)] is...
Text Solution
|
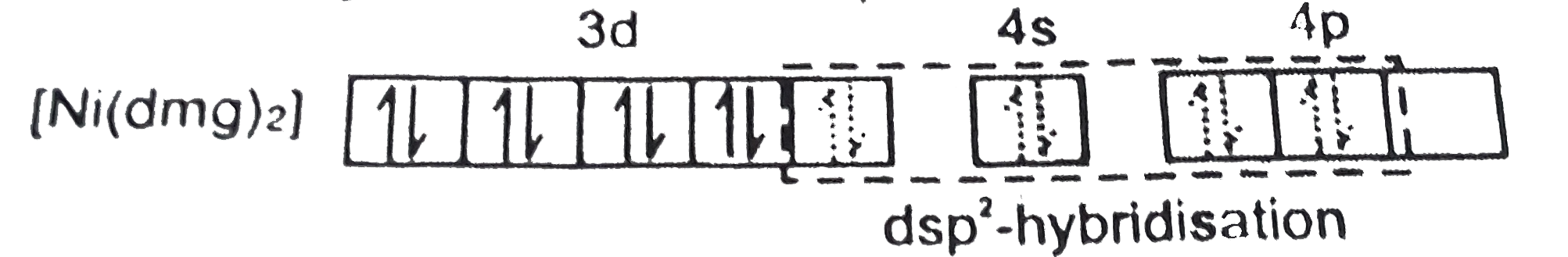
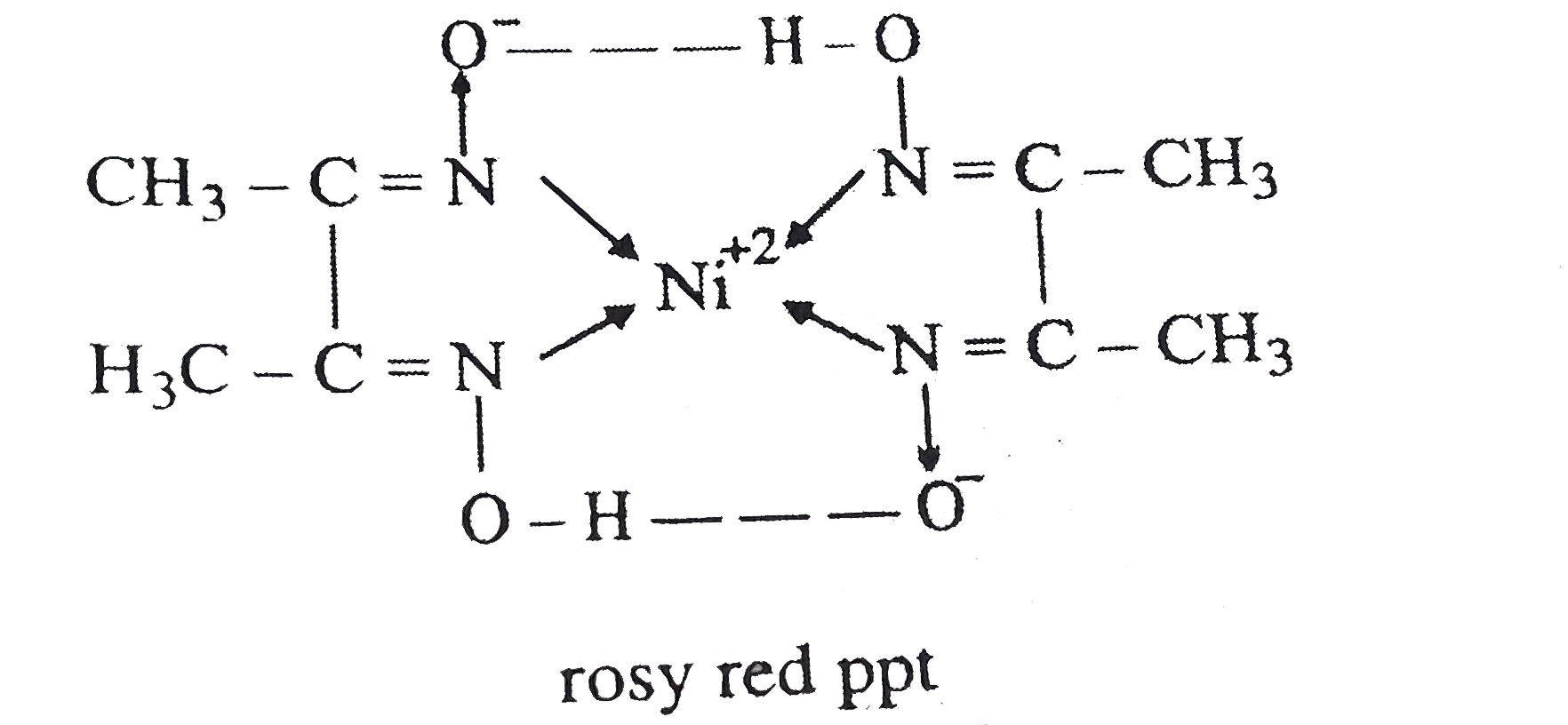
- When diemethyl glyoxime is added to the aqueous solution of nickel (I...
Text Solution
|
- Which kind of isomerism is exhibited by octahedral [Co(NH(3))(4)Br(2)]...
Text Solution
|
- The bond length is CO is 1.128Å what will be the bond length of CO in ...
Text Solution
|
- NiCl(2) overset(KCN)to "complex"A NiCl(2) underset("excess") overset...
Text Solution
|
- NiCl(2) overset(KCN)to "complex"A NiCl(2) underset("excess") overset...
Text Solution
|
- Among the following metal carbonyls , the C-O bond order is lowest in ...
Text Solution
|
- Match the complexes in Column-I with their properties listed in Column...
Text Solution
|
- The IUPAC name of [Ni (NH(3))(4)] [NiCl(4)] is
Text Solution
|
- Both [Ni(CO)(4)] and [Ni(CN)(4)]^(2-) are diamagnetic. The hybridisati...
Text Solution
|
- Statement-1: The geometrical isomers of the complex [M(NH(4))(4)Cl(2)]...
Text Solution
|
- Statement I [Fe(H(2)O)(5)NO]SO(4) is paramagnetic Statement II The F...
Text Solution
|
- The compound (s) the exhibit(s) geometrical isomerism is (are ) :
Text Solution
|
- The spin only magnetic moment value ( in Bohr magneton units ) of Cr(C...
Text Solution
|
- The correct structure of ethylenediamineteraacetic acid (EDTA) is .
Text Solution
|
- The ionisation isomer of [Cr(H(2)O)(4) Cl (NO(2))]Cl
Text Solution
|
- The complex showing a spin -magnetic momnet of 2.82 BM is .
Text Solution
|
- Total number of geometrical isomers for the complex [Rh Cl (CO) (PPh(3...
Text Solution
|
- Geometrical shapes of the complex formed by the reaction of Ni^(2+) wi...
Text Solution
|