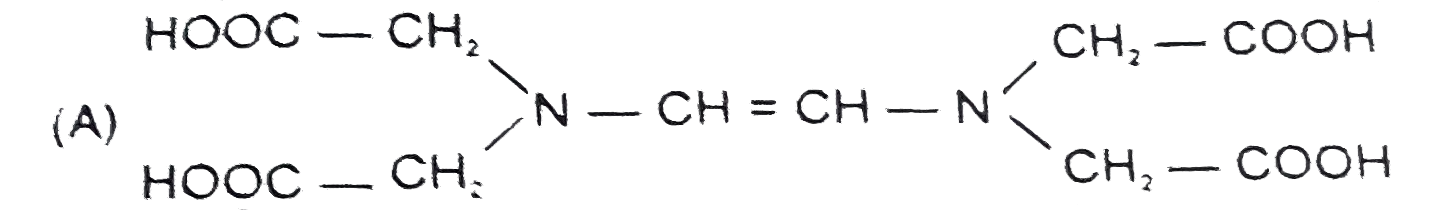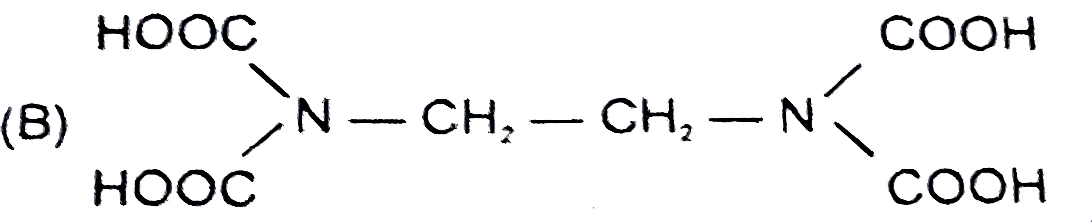A
B
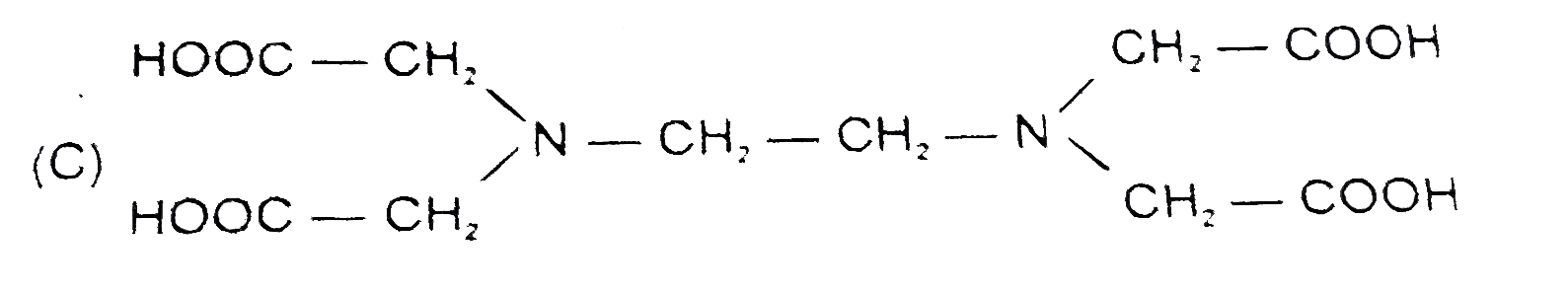
C
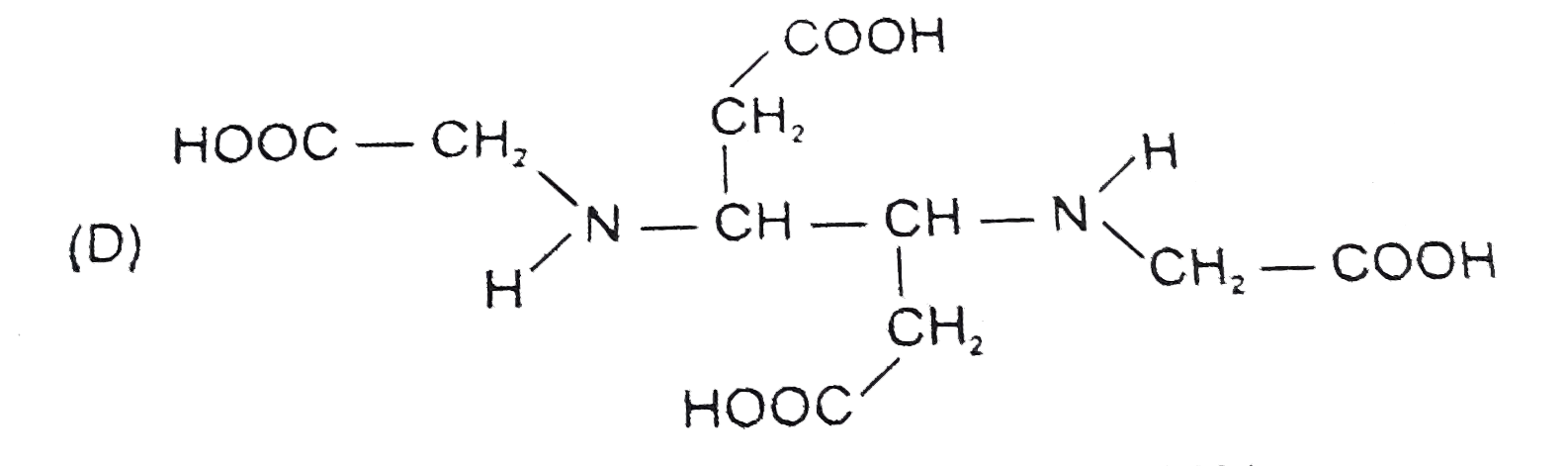
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE|Exercise Exercise-3 Part-I: JEE(Advance ) /IIT-JEE Problem (Comprehenion )|1 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Exercise-3 Part-II: JEE(Main ) /AIEEE Problem|36 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Exercise-2 Part-IV: Comprehension -5|1 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(d & f- Block Elments)|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-COORDINATION COMPOUNDS-Exercise-3 Part-I: JEE(Advance ) /IIT-JEE Problem
- The compound (s) the exhibit(s) geometrical isomerism is (are ) :
Text Solution
|
- The spin only magnetic moment value ( in Bohr magneton units ) of Cr(C...
Text Solution
|
- The correct structure of ethylenediamineteraacetic acid (EDTA) is .
Text Solution
|
- The ionisation isomer of [Cr(H(2)O)(4) Cl (NO(2))]Cl
Text Solution
|
- The complex showing a spin -magnetic momnet of 2.82 BM is .
Text Solution
|
- Total number of geometrical isomers for the complex [Rh Cl (CO) (PPh(3...
Text Solution
|
- Geometrical shapes of the complex formed by the reaction of Ni^(2+) wi...
Text Solution
|
- Among the following complexes : K(3)[Fe(CN)(6)],[Co(NH(3))(6)]Cl(3) , ...
Text Solution
|
- The volume (in mL) of 0.1M Ag NO(3) required for complete precipitatio...
Text Solution
|
- As per IUPAC nomenclature, the name of the complex [Co(H(2)O)(4) (NH(3...
Text Solution
|
- [NiCl(2){P(C(2)H(5))(2)(C(6)H(5))}(2)] exhibits temperature dependent ...
Text Solution
|
- Consider the follwing complexes ion P,Q and R P =[FeF(6)]^(3-), Q=[V...
Text Solution
|
- The pair of coordination complex exhibiting the same kind of isomerism...
Text Solution
|
- EDTA^(4-) i9s ethylenediamine tetraacetate ion The total number of N-C...
Text Solution
|
- A list of species having the formula of XZ(4) is given below XeF(4), S...
Text Solution
|
- Match each coordination compounds in List-I with an appropriate pair o...
Text Solution
|
- For the octahedral complexes of Fe^(3+) in SCN^(-)(thiocyanato-S) and ...
Text Solution
|
- In the complex acetylbromidodicarbonylbis (triethylphosphine) iron (II...
Text Solution
|
- Among the complex ions, [Co(NH(2) - CH(2) - CH(2) - NH(2))(2) Cl(2)...
Text Solution
|
- Among [Ni(CO)(4)], [NiCl(4)]^(2-), [Co (NH(3))(4) Cl(2)] Cl, Na(3) [C...
Text Solution
|