A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-II|68 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-III Subjected Question|7 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Exercise-3 Online Exam|22 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(d & f- Block Elments)|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-COORDINATION COMPOUNDS-Additional Problem for Self Practice (APSP) Part-I
- Which complex is likely to show optical activity :
Text Solution
|
- Which kind of isomerism is shown by the complex [Co(NH(3))(5)(ONO)]SO(...
Text Solution
|
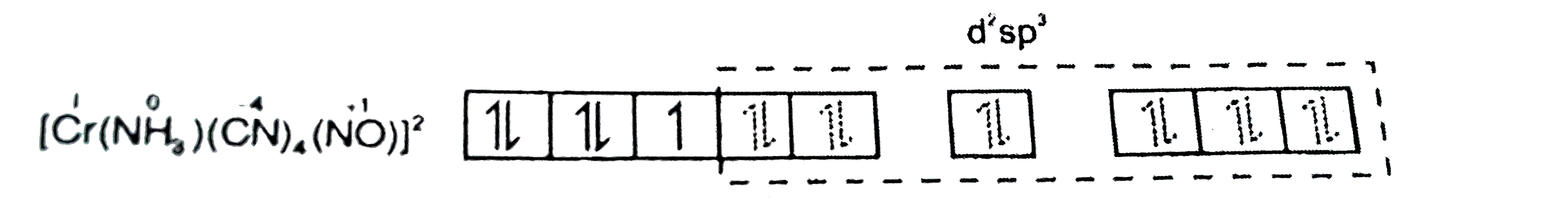
- Which of the following statements is correct for complex [Cr(NH(3))(CN...
Text Solution
|
- S-1 : [Cr(NH(3))(6)]^(3+) is a inner orbital complex with S-2: The c...
Text Solution
|
- Which of the following statement is false ?
Text Solution
|
- If excess of AgNO(3) solution is added to 100mL of a 0.024 M solution ...
Text Solution
|
- A complex of certain metal has the magnetic moment of 4.91 BM whereas ...
Text Solution
|
- Oxidation number of Fe in violet coloured complex Na(4)[Fe(CN)(5)(NOS)...
Text Solution
|
- Aqueous solution of nickel sulphate on treating with pyridine and then...
Text Solution
|
- The IUPAC name of [Co(NH(3))(6)][Cr(C(2)O(4))(3)] is :
Text Solution
|
- In the compound lithiumtetrahydroaluminate, the ligands is :
Text Solution
|
- The oxidation numbe of Co in the complex ion is :
Text Solution
|
- The magnitude of crystal field stabilisation energy (CFSE of Delta(1))...
Text Solution
|
- Other than the X-ray difference , how could be the following pairs of ...
Text Solution
|
- [Fe(en)(2)(H(2)O)(2)]^(2+) +en to " complex(X)". The correct statement...
Text Solution
|
- Which of the following pairs will show the same magnetic moment ('spin...
Text Solution
|
- What will be the 'spin only' magnetic moment of the complex formed whe...
Text Solution
|
- Which of the following statement about Fe(CO)(5) is correct ?
Text Solution
|
- The crystal field -splitting for Cr^(3+) ion in octahedral field chang...
Text Solution
|
- Which of the following complex ion is not expected to absob visible li...
Text Solution
|