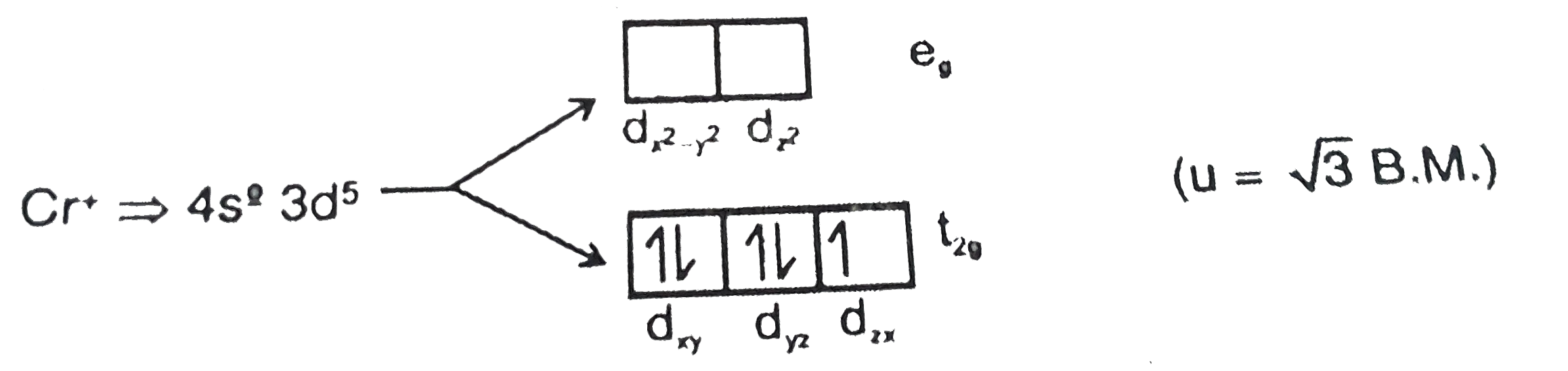Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-III One or more than one option correct type|7 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-III Comprehension.|3 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-III Match the column|1 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(d & f- Block Elments)|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-COORDINATION COMPOUNDS-Additional Problem for Self Practice (APSP) Part-III Single and double value integer type
- What is the coordination number of metal in [M("trien")("dipy")]^(pmn)...
Text Solution
|
- Out of the following. How many have correct IUPAC naming :- (1) [Ni(...
Text Solution
|
- How many of the given complexes follow E.A.N rule ? (a) [Fe(CO)(5)]...
Text Solution
|
- A name of neutral complex is : Bis (acetyl acetanato) methylcyanido...
Text Solution
|
- Na(2)[Cr(NO)(NH(3))(C(2)O(4))(2)], u=sqrt(3)BM, Then total no. of elec...
Text Solution
|
- If CFSE increases by 30% and 40% respectively for Co^(3+) " to " Rh^(3...
Text Solution
|
- For the [Cr(H(2)O)(6)]^(2+) ion, the mean pairing energy P is found to...
Text Solution
|
- The possible number of stereoismers for the formula [Ma(2)b(2)cd]^(pmn...
Text Solution
|
- A complex is prepared by mixing CoCl(3) & NH(3) 0.1 M solution of the...
Text Solution
|
- Calculate total number of geometrical, optical and structural isomers ...
Text Solution
|
- What is the EAN value of W(CO)(6) carbonyl ompounds ?
Text Solution
|