Text Solution
Verified by Experts
Topper's Solved these Questions
NITROGEN & OXYGEN FAMILY
RESONANCE|Exercise Exercise 1|41 VideosNITROGEN & OXYGEN FAMILY
RESONANCE|Exercise Exercise 1 (Part-II : Only one option correct type)|50 VideosMETALLURGY
RESONANCE|Exercise INORGANIC CHEMISTRY(Metallurgy)|42 VideosNITROGEN CONTAINING COMPOUNDS
RESONANCE|Exercise ORGANIC CHEMISTRY(Nitrogen containing Compounds)|30 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-NITROGEN & OXYGEN FAMILY-Part-III Practice Test-2 (JEE (Advanced Pattern))
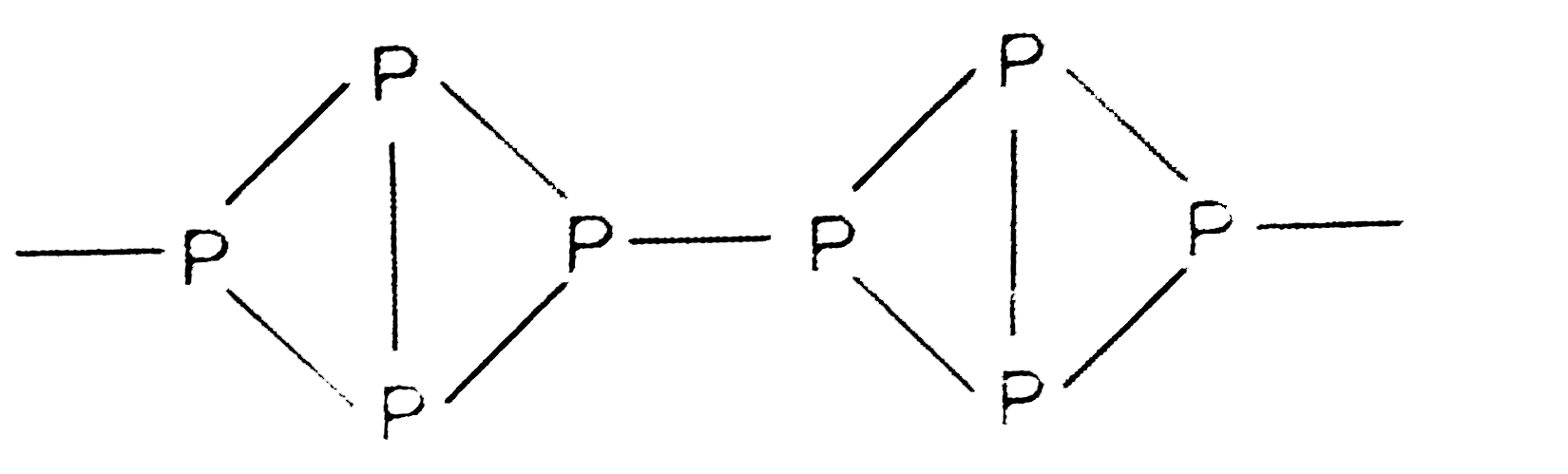
- Explain the high reactivity of white phosphorus as compared to red pho...
Text Solution
|
- An unknown substance (P) functions as weak base in water. It produces ...
Text Solution
|
- The compound which gives oxygen on moderate heating is
Text Solution
|
- Which of the following compounds does give N(2) on heating ?
Text Solution
|
- Which of the following cannot result in the formation of NO ?
Text Solution
|
- A gaseous substance dissolve in water giving a pale blue solution whic...
Text Solution
|
- Which of the following will not decolourise acidified KMnO(4) ?
Text Solution
|
- Which of the following statements is not true about ozone ?
Text Solution
|
- Sulphuric acid reacts with PCl5 to give
Text Solution
|
- Which is greater for P(4) (white) tan P(4) (red)
Text Solution
|
- What is//are not true abut phosphine (PH(3))?
Text Solution
|
- Which of the following is/are correct regarding nitrogen family
Text Solution
|
- P(2)O(5) can dehydrate
Text Solution
|
- What products are formed when H(3)PO(2) is heated at 415 K and at 435 ...
Text Solution
|
- 4AgNO(3) + 2H(2)O + H(3)PO(2) overset("boil")rarr 4 Ag + 'X' + 'Y' I...
Text Solution
|
- Cold dilute nirtic acid would dissolved how many of the following with...
Text Solution
|
- Which of the following on heating will produce an oxide of nitrogen ...
Text Solution
|
- NaPO(3) can significantly react with how many of the following ? CaC...
Text Solution
|
- One mole of PCl(3) is dissolved in excess of water. No. of moles of Na...
Text Solution
|
- When hypo solution react with CuSO(4) and produce soluble complex, the...
Text Solution
|
- How many of the following reaction yield POCl(3) ? {:((i) PCl(3) + O...
Text Solution
|