Text Solution
Verified by Experts
Topper's Solved these Questions
NITROGEN & OXYGEN FAMILY
RESONANCE|Exercise Exercise 1 (Part-II : Only one option correct type)|50 VideosNITROGEN & OXYGEN FAMILY
RESONANCE|Exercise Exercise 1 (Part-III: Match the column)|2 VideosNITROGEN & OXYGEN FAMILY
RESONANCE|Exercise Part-III Practice Test-2 (JEE (Advanced Pattern))|23 VideosMETALLURGY
RESONANCE|Exercise INORGANIC CHEMISTRY(Metallurgy)|42 VideosNITROGEN CONTAINING COMPOUNDS
RESONANCE|Exercise ORGANIC CHEMISTRY(Nitrogen containing Compounds)|30 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-NITROGEN & OXYGEN FAMILY-Exercise 1
- Write the following for a white phosphorus molecule (a) oxidation st...
Text Solution
|
- Tellurium forms oxides of the formula TeO, TeO(2) and TeO(3). What is ...
Text Solution
|
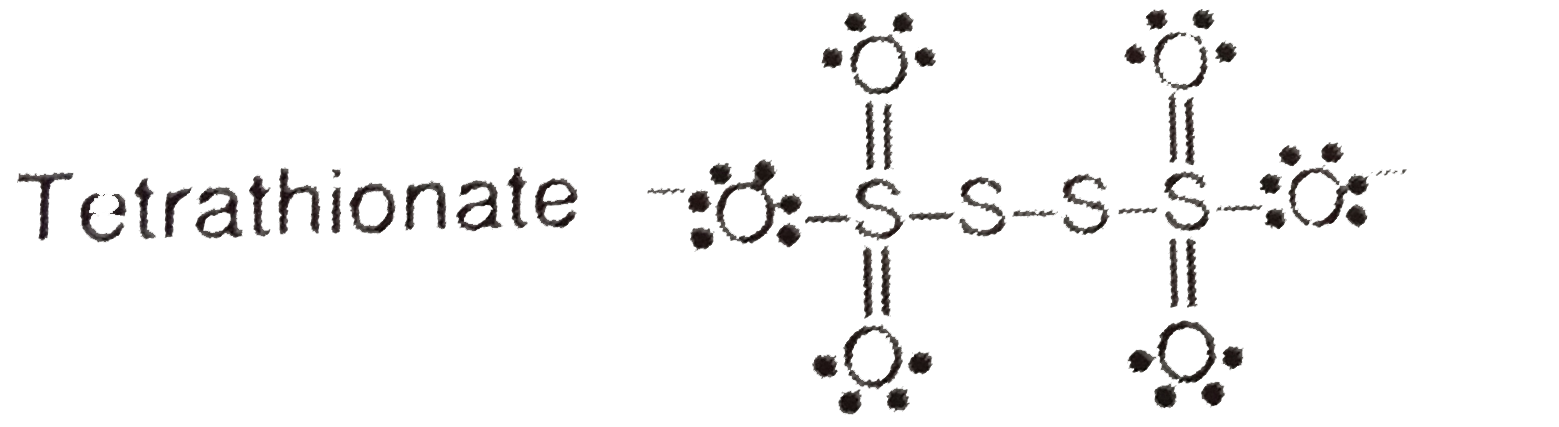
- Write the structure and oxidation number of sulphur in tetrathionate i...
Text Solution
|
- Bond angle in PH(4)^(+) is higher than that in PH(3).Why ?
Text Solution
|
- Write the oxyacids of the following {:("Oxide","Oxyacids"),(N(2)O(3)...
Text Solution
|
- N(2), CO, CN^(-) and NO^(+) are isoelectronic but the former is chemic...
Text Solution
|
- On moving down the group from O to Te acidic strength increases, why ?
Text Solution
|
- What happens when barium azide is heated ?
Text Solution
|
- Which stable elements of 15^(th) and 16^(th) group do not react with w...
Text Solution
|
- Chemiluminescence is a phenomenon in which on element glows in dark wh...
Text Solution
|
- Among the hydrides of group 16, water shows unsual boiling point. Why ...
Text Solution
|
- Ammonium salts generally resemble those of postassium and rubidium in ...
Text Solution
|
- Write balanced equation when NH(3) is dissolves in (a) water (b) HCl...
Text Solution
|
- What happens when phosphine is absorbed in mercuric chloride solution ...
Text Solution
|
- On being slowly passed through water, PH(3) forms bubbles but NH(3) di...
Text Solution
|
- How is hydrazine prepared ?
Text Solution
|
- Both PH(3) and NH(3) are Lewis bases, but basic strength of PH(3) is l...
Text Solution
|
- In the preparation of P(4)O(6), a mixture of N(2) and O(2) is used rat...
Text Solution
|
- A compound of 15^(th) group element is used as a fast drying agent in ...
Text Solution
|
- Write the structures of the oxides: N(2)O(3), N(2)O(5)
Text Solution
|